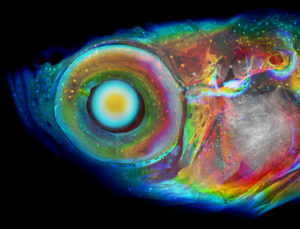
A zebrafish is shown imaged using a new CT method, with colors assigned to structures based on their depth within the fish. Learn more about the method.
The Zebrafish Functional Genomics Core at the Penn State College of Medicine was established to provide the Penn State research community with a modern, centralized facility for housing, breeding and performing experiments with zebrafish, one of the fastest-growing model systems in biomedical research.
The mission of the core is to provide a physical and intellectual infrastructure for investigators whose research can benefit from use of the zebrafish as a model system.
The zebrafish core project was supported, in part, by the Office Of The Director of the National Institutes Of Health under Award Number G20OD016619. It is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco CURE Funds. The Department specifically disclaims responsibility for any analysis, interpretations or conclusions.
Additional support was generously provided by The Jake Gittlen Laboratories for Cancer Research and Penn State College of Medicine’s Department of Pathology, Institute for Personalized Medicine, Department of Neurology, Department of Neurosurgery and Department of Pediatrics.
Jump to topic
Search
Instrumentation and Services

A rack of Pentair Aquatic Habitats are seen in the zebrafish core.
- A newly designed facility housing a state-of-the-art Pentair Aquatic Habitats recirculating aquaria, located in the Department of Comparative Medicine.
- A central housing room equipped with 32 racks of recirculating aquaria, able to accommodate any combination of one-, three- and 10-liter tanks.
- Twin, 16-rack recirculating systems equipped with independent filtration/purification, allowing for individualized control of system water quality and conditions.
- An isolated quarantine room containing three stand-alone housing systems.
- A sentinel program monitoring each system for the presence of pathogens and prevent the spread of disease.
- A procedure room containing four micro injection stations and two light-tight photo booths to provide bright field and fluorescent imaging.
- Three independently controlled light-tight breeding cabinets.
- Mechanical filtration: Each of the recirculating systems in the core is outfitted with its own self-cleaning, rotating drum filter equipped with a 40-micron screen. Removing particulate waste early in the filtration process allows for increased efficiency of bio-filtration and UV sterilization, both critical steps in preventing the over proliferation of microbial pathogens.
- Biological filtration: The core uses the Kaldnes Moving Bed biofiltration system, in which buoyant polyethylene biomedia is kept in continuous motion allowing for the exfoliation of older, less active bacteria.
- Chemical filtration: Activated carbon removes dissolved chlorine/chloramine and organic compounds from source water.
- UV disinfection: Ultraviolet germicidal irradiation units add an additional level of sterilization to pre-filtered water. The system utilizes germicidal ultraviolet radiation type C, with wavelengths between 200 and 280 nm, the most effective range for sterilization. The minimum performance of each unit is 1000 μW/cm2.
- Four stations with dedicated microinjectors, manual micromanipulators and stereo microscopes provide ease of scheduling.
- Eppendorf FemtoJet microinjectors provide reproducible injection of volumes ranging from microliters to femtoliters.
- Manual micromanipulators allow needle positioning along three axes, with .01 mm precision along the X axis.
- ZEISS Stereo Discovery.V8 microscopes provide gentle, uniform illumination for zebrafish embryo microinjection and sorting.
- 1:8 zoom ratio
- Magnification from 10X to 80X
- Brightfield and darkfield with oblique-light contrast
- Leica MZ FLIII Fluorescence Stereomicroscope for rapid, non-destructive 3D microscopy and digital imaging of fluorescing specimens.
- Magnification from 8X to 100X
- Equipped with 1X and 1.6X planaopochromatic objectives
- Filters: Cy3TM, GFP2, GFP4, Texas Red and TRITC
- Olympus MVX10 MacroView Macro Zoom Fluorescence Microscope designed specifically for fluorescence imaging.
- Equipped with 1X MVX Plan Apochromat Lens
- MVX10 objectives produce minimal autofluorescence
- Long working distance allows observation at optimum magnification
- Short exposure time ensures maximum specimen protection
- ZEISS Axio Zoom.V16 Zoom Fluorescence Microscope with ZEISS ApoTome.2 provides superior widefield brightfield, darkfield and fluorescent imaging.
- 1:16 zoom ratio with magnification from 7X to 250X
- Plan NeoFluar 1.0x and Plan NeoFluar 2.3x objectives
- Motorized stage (XY) and focus (Z)
- Filter sets: 38HE (GFP), 49HE (DAPI), and 64HE (Texas Red)
- Equipped with ZEISS ApoTome.2 for widefield fluorescent imaging and optical sectioning for 3D rendering
- Outfitted with AxioCam 503 Color and Monochrome cameras
Procedures, Protocols and Forms
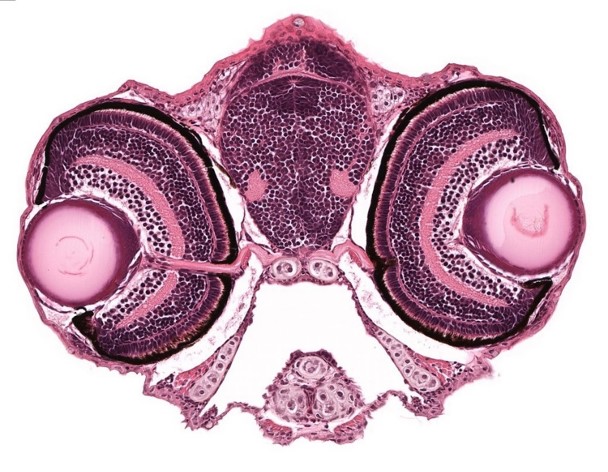
A stained transverse histological section of 5 dpf WT larva is seen in an image from the Zebrafish Atlas.
A long-established model of developmental biology, due to its optical clarity, fecundity and regenerative capabilities, the zebrafish (Danio rerio) has become one of the preeminent models of human genetic disease, thanks in part to the availability of a high-quality, annotated genetic sequence and targeted genome editing tools such as TALENs and CRISPR/Cas9 that can be used to rapidly generate stable transgenic lines through reverse genetics.
Approximately 70 percent of human protein-coding genes and 84 percent of human disease-associated genes have functional genetic homologs in zebrafish.
These features, combined with the relative economy of the model, have secured a place for zebrafish among the classical model species.
Regulations dictate that all investigators wishing to use the Zebrafish Functional Genomics Core are required to submit or amend the necessary documentation prior to beginning any research project. These include the following:
Occupational Health and Safety Program
Prospective animal handlers must complete the Occupational Health and Safety Program administered by Employee/Student Health. Appointments can be scheduled through Employee Health by calling 717-531-8280 or Student Health by calling 717-531-5998 Three forms – Initial Health Review, Job Profile and Annual Review – must be completed and taken to the appointment. All three forms can be found on the Animal Resources section of the Infonet. Upon completion of the program, participants will receive a feedback summary that must be faxed to Rachel Panas in the IACUC Compliance Office at 717-531-5001 or emailed to rpanas@pennstatehealth.psu.edu. Investigators should send an additional copy of the summary to Peggy Hubley in the zebrafish core at pjh11@psu.edu.
Institutional Animal Care and Use Committee (IACUC) Animal Protocol Form
An IACUC Animal Use Protocol Form must be submitted by any investigator using laboratory animals. Investigators receiving training or taking part in pilot projects not supported by external funding may be added as amendments to the facilities’ existing protocol. (Questions regarding the Pilot/Training program should be directed to Khai C. Ang at kca2@psu.edu). Note that IACUC requires investigators working with fish to complete two online courses, “Introduction to Fish” and “Working With Laboratory Zebrafish,” available through the American Association for Laboratory Animal Science Learning Library, and pass the quiz at the end of the module. See instructions for using AALAS Learning Library (Penn State Access ID login required).
Biological Safety and Recombinant DNA (BSRD) Assurances Forms
Biohazardous materials and recombinant DNAs used to generate transgenic organisms must receive approval from the Biosafety and Recombinant DNA (BSRD) Committee prior to transport or use.
Amendments to existing BSRD forms should be made in a text color different from that on the existing form. This allows for faster review and approval, as well as providing a means of tracking changes. New or amended BSRD forms should be sent to biosafetyofficer@pennstatehealth.psu.edu. Additional information can be found on the Biosafety Resources section of the Infonet (internal access only; login required).
Once the above documentation is in place, investigators must contact Peggy Hubley in the core to schedule hands-on training in fish husbandry and core procedures by emailing pjh11@psu.edu.
Once complete, investigators will be given keys to the core.
Additionally, investigators will need to contact Penny Devlin, Animal Resource Facilities Manager for the Department of Comparative Medicine, at pdevlin@pennstatehealth.psu.edu for a brief introduction to the Central Animal Quarters and to obtain keycard access to that facility.
Any investigator wishing to import embryos or adult fish into the core must obtain written consent from the fish facility manager, Peggy Hubley, before proceeding. She can be reached at pjh11@psu.edu.
Once written consent to import has been obtained, the following actions must be taken:
- An Animal Import Request Form must be obtained from and submitted to Peggy Hubley.
- A health certificate must be issued by the sending institution and submitted to Peggy Hubley. A health certificate template can be provided.
- Incoming fish must be added to an IACUC animal protocol. New researchers without their own IACUC animal protocol must place incoming fish on a holding protocol, held by the Department of Comparative Medicine, until the new researcher procures their own IACUC animal protocol. Contact Rachel Panas at rpanas@pennstatehealth.psu.edu for assistance with the IACUC Animal Protocol Form.
- Shipments should be scheduled to arrive no later than Thursday morning.
- All fish must be labeled with date of birth and genotype. An identification number will be assigned to each group of fish in accordance with facility guidelines.
- Adult fish entering the facility will be housed in quarantine for the duration of life; contact Peggy Hubley for quarantine pricing.
- All embryos must be bleached prior to shipment in order to be entered into the main housing facility. Unbleached embryos will remain in quarantine. See the procedures for shipping live adult fish and embryos for the accepted embryo bleaching protocol.
- A Material Transfer Agreement is not needed unless:
- the exporting institution requires one;
- the original owner of the animal model or reagents requires a new MTA with Penn State; or
- the researcher is transferring data or tissues related to human subjects.
The Department of Comparative Medicine governs the exportation of all laboratory animals from Penn State College of Medicine to other facilities. The following guidelines must be followed when exporting live embryos or adult fish from the zebrafish core to any other institution:
- An Animal Export Request Form must be obtained from and submitted to Peggy Hubley. She can be reached at pjh11@psu.edu.
- A health certificate must be obtained from a Penn State-certified veterinarian via Peggy Hubley.
- A Material Transfer Agreement is not needed unless:
- the importing institution requires one;
- the original owner of the animal model or reagents requires a new MTA with Penn State; or
- the researcher is transferring data or tissues related to human subjects.
- An invoice/packing slip is required.
- There must be appropriate labeling on bags and boxes.
Shipment of embryos or adult fish from the core facility will be performed by facility manager, Peggy Hubley, via FedEx overnight.
The zebrafish core is certified by FedEx to ship live embryos and adult fish and will do so for any investigator housing fish within the facility. Strict guidelines must be followed when packaging embryos and fish.
The procedures below were adapted from ZFIN by Peggy Hubley in 2015; additional information and images are available online through Zebrafish Information Network (ZFIN).
Supplies Needed
- Styrofoam box securely nested in a cardboard box that is rated at 44 ECT crush strength (see examples below)
- Plastic bags (2 mil) for adult fish or tissue culture flasks for embryos
- Rubber bands
- Labels: FedEx address label, detailed labels for fish bags, two LIVE FISH labels and two DO NOT X-RAY labels for outer box (see examples below)
- Packing: foam peanuts (avoid using dissolvable peanuts) or bubble wrap
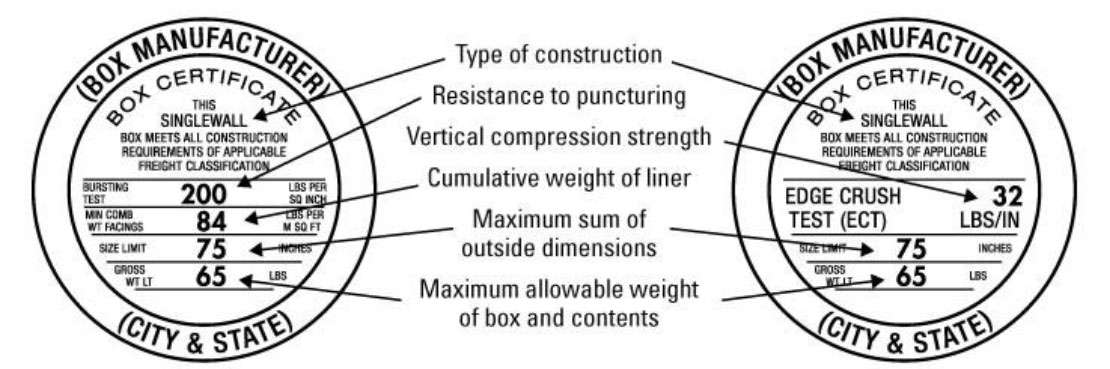
This image shows how to determine the Edge Crush Test (ECT) strength of a box. Shipping zebrafish requires the use of a box rated for at least 44 lbs/in ECT.

When shipping zebrafish, investigators must include two LIVE FISH labels and two DO NOT X-RAY labels for the outer box, as seen in this example.
Packing Adult Fish
Note: Adult fish are shipped in water filled plastic bags, at five fish per bag.
- Label bags with detailed information (genotype, line, number, age, etc.)
- Fill bag one-third full with aquarium water. Add fish.
- In order to trap as much air as possible, arrange the water filled bag to be as open as possible. Grasp the top of the bag, closing it off and twisting. Twist well, keeping the pressure in the bag so that it looks like a cushion. Check the bag at this point to see that it is two-thirds air, one-third water. Do not blow into the bag to add more air; open the bag and try to capture more.
- Close off the bag by either knotting it or twisting the bag so that the twist doubles over and rubber band this portion. Push the closure down to maintain the pressure in the bag. The bag should remain tight. Take the bottom points of the bag and tape them up along the sides of the bag, creating a square bottom. This prevents fish from getting caught and injured in the corners.
- Place the bag into a second plastic bag going the other direction, double-bagging it. Close off the outer bag by knotting it or rubber-banding it.
- Place the bagged fish in the shipping box, and fill up any space with Styrofoam packing peanuts.
Protocol for Shipping Embryos
- Fill a 250 ml tissue culture flask with 150 ml of sterile (filtered or autoclaved) fish system water.
- Add 100 bleached embryos per flask (see directions for bleaching eggs below). Preferably send one-day-old fish. Flask should have air space, and not be filled entirely with fluid.
- Close lid tightly. Seal lid with Parafilm.
Bleaching Eggs
- Prepare a solution of bleach water by adding 59 μl (0.059 ml) of 5.25 percent sodium hypochlorite (bleach) to 100 ml of system water in a beaker.
- Decant as much water as possible from eggs to be bleached.
- Add bleach solution and gently swirl to separate the eggs, allowing the entire surface of each chorion to come into contact with the bleach solution.
- After two minutes in the solution, decant the bleach solution.
- Add sterile (filtered or autoclaved) fish system water.
- Again, swirl the eggs and allow each one to receive a thorough rinse.
- Repeat this rinse once or twice more.
Boxing Fish
- Add a heat pack and/or ice pack when temperatures are extreme.
- Place the inner Styrofoam lid on the inside Styrofoam box. This inside lid helps insulate the fish.
- Taping the lid all around serves to prevent water from leaking outside of the box should an accident occur.
- Tape can be added to all of the seams of the cardboard box to help contain possible leaks.
- The address label is attached to the top of the box, where customs papers would also be added for international shipments.
- Live fish labels for the outside of the box can be obtained from Peggy Hubley or from Zebrafish Information Network (ZFIN).
- Zebrafish International Resource Center (ZIRC)
- Zebrafish Information Network (ZFIN)
- Zebrafish Atlas, part of Penn State’s Bio-Atlas at the Jake Gittlen Laboratories for Cancer Research
- Ensembl Zebrafish Genome Browser
- University of California Santa Cruz Zebrafish Genome Browser
- Zebrafish Microinjection Techniques (video) by JoVE: The Journal of Visualized Experiments
All publications, press releases or other documents that result from the utilization of any Penn State College of Medicine Institutional Research Resources, including funding, tools, services or support, are required to credit the core facility and associated RRID.
“The Zebrafish Functional Genomics Core (RRID:SCR_021199) services and instruments used in this project were funded, in part, by the National Institute of Health (1G20OD016619-01), the Pennsylvania State University College of Medicine via the Office of the Vice Dean of Research and Graduate Students and the Pennsylvania Department of Health using Tobacco Settlement Funds (CURE). The content is solely the authors’ responsibility and does not necessarily represent the official views of the University or College of Medicine. The Pennsylvania Department of Health disclaims responsibility for any analyses, interpretations, or conclusions.”
The following boilerplate language can be used in grant applications when referencing the College of Medicine Zebrafish Functional Genomics Core.
The zebrafish (Danio rerio) – a long-established model of developmental biology due to its optical clarity, fecundity, small size, economical husbandry and regenerative capabilities – has become one of the preeminent models of human genetic disease, thanks in part to the availability of a high-quality, annotated genetic sequence and targeted genome editing tools such as TALENs and CRISPR/Cas9 that can be used to rapidly generate stable transgenic lines through reverse genetics. These features make it suitable not only for mutagenesis screens, but also for targeted mutagenesis high-throughput whole-animal imaging and drug screens. Approximately 70 percent of human protein-coding genes and 84 percent of human disease-associated genes have functional genetic homologs in zebrafish. These features, combined with the relative economy of the model, have secured a place for zebrafish among the classical model species.
The Zebrafish Functional Genomics Core at Penn State College of Medicine was established to provide Penn State’s research community with a modern, centralized facility for housing, breeding and performing experiments with zebrafish, as well as the intellectual infrastructure to do that work. The core totals 1,242 net square feet and features a state-of-the-art Pentair Aquatic Habitats tank system. The central housing room (total net space of 975 square feet) features twin 16-rack recirculating systems equipped with independent filtration/purification, allowing for individualized control of system condition and water quality.
The filtration system comprises
- mechanical filtration outfitted with rotating drum filter equipped with a 40-micron screen
- biological filtration that uses the Kaldnes Moving Bead bio-filtration with buoyant polyethylene bio-media to keep continuous water motion and allowing for exfoliation of older, less active bacteria
- chemical filtration with activated carbon to remove dissolved chlorine/chloramine and other organic compounds
- UV disinfection that utilizes germicidal ultraviolet radiation type C, with wavelengths between 200 and 280 nm for the most effective range of sterilization
The recirculating aquaria are able to accommodate any combination of one-, three- and 10-liter tanks, and the water temperature is maintained at 28 degrees C. In addition, the core is also equipped with two genotyping tanks and two Tecniplast Z-park mass breeding tanks. It also houses three independently controlled light-tight breeding cabinets for control embryo production to maximized experiment efficiency. An isolated quarantine room (91 net square feet) containing three stand-alone housing systems allows researchers to import fish into the facility without jeopardizing the welfare of the central housing facility. The controlled lighting in the core simulates dawn and dusk, and room temperature of the core is set at 25.5 degrees C year-round.
The adjacent procedure room (net space of 176 square feet) contains four Eppendorf microinjection stations (one FemtoJet express, two FemtoJet and one Femtojet 4i pressurized by an external air compressor), four Zeiss Stereo Discovery V8 microscopes and one Zeiss Stemi308. The Stemi 308 can be connected through Bluetooth to portable devices, and also to an OLED TV for training and education. The core also equipped with two photo booths, providing bright field and fluorescent imaging. One of the photo booths houses an AxioZoom fluorescent microscope with ApoTome that provides superior widefield brightfield, darkfield and fluorescent imaging. The Zeiss ApoTome is capable of optical sectioning for 3-D rendering. The microscope is outfitted with AxioCam 503 color and monochrome cameras and has the capability for Z-stack and tile imaging through the motorized stage. Another photo booth houses an Olympus SZX10 fluorescent microscope. The core has also housed a zebrafish behavior monitoring and imaging system from Noldus that is capable of tracking up to 96 fish simultaneously, equipped with tapping and light simulation, and EthoVision visualization and analysis software. The core also has one freezer, one refrigerator, two incubators for embryo development, one dishwasher, two brine shrimp/rotifer systems, one P-97 Sutter Instruments needle puller and three computers.
More information about the core is available at https://research.med.psu.edu/core-facilities/zebrafish.
Work With the Zebrafish Core
Equipment
The core’s goal is to support the Penn State research community. To that end, unassisted use of microinjection stations, microscopes and imaging equipment is free of charge to researchers housing fish in the facility. However, as improper use of equipment can easily result in damage and weeks of lost productivity while waiting for repair, all new users are required to complete an initial one-on-one training session with additional consultation available as needed. Unassisted use of equipment will be permitted only after users have demonstrated proficiency to a member of the core facility staff.
Fish
Fish are available to both Penn State and external investigators. The core currently houses the following wild type lines: AB, Conner, Ekkwill, Liles, Tübingen (Tu) and WIK. It also houses a selection of mutant and transgenic lines.
Housing
Tank fees are charged per whole/half rack on a monthly basis. One whole rack can hold a maximum of 100 x 1L, 60 x 3L or 30 x 10L tanks. For example, one whole rack can hold 40 x 1L, 24 x 3 and 6 x 10L tanks. Tank sizes can be substituted as needed to meet the changing requirements of a research project. Additionally, a limited number of 10-gallon tanks are available.
Fish may be purchased directly from the core or they may be acquired from an outside source (ZIRC, collaborating investigators, etc.). Please note, however, that only bleached eggs can be introduced into the primary facility. Any adult fish acquired from an outside source must remain in the quarantine facility for the duration of their life.
For current pricing or availability of fish or housing, contact the core.
Listed here are some recent publications related to the field of zebrafish research from a variety of institutions.
