The Pulmonary Immunology and Physiology Core at Penn State College of Medicine aids investigators in the development and testing of small animal models to be used for pre-clinical pulmonary research.
It is a BSL2 facility that allows the assessment of pulmonary function related to many clinical situations, including airway hyper-responsiveness, ventilation-induced lung injury, postnatal lung development, airway remodeling, chronic inflammation, allergy, environmental exposures and infection. In addition, services offered include oxygen profiling, infection and treatment; the ability to deliver, measure and test the pre-clinical potential of therapeutic interventions via aerosol; and the housing of small animals used in these studies.
The Pulmonary Immunology and Physiology Core is located in the Department of Comparative Medicine (Room CG737) and is operated under the oversight of the Department of Pediatrics.
Jump to topic
Search
Instrumentation and Services
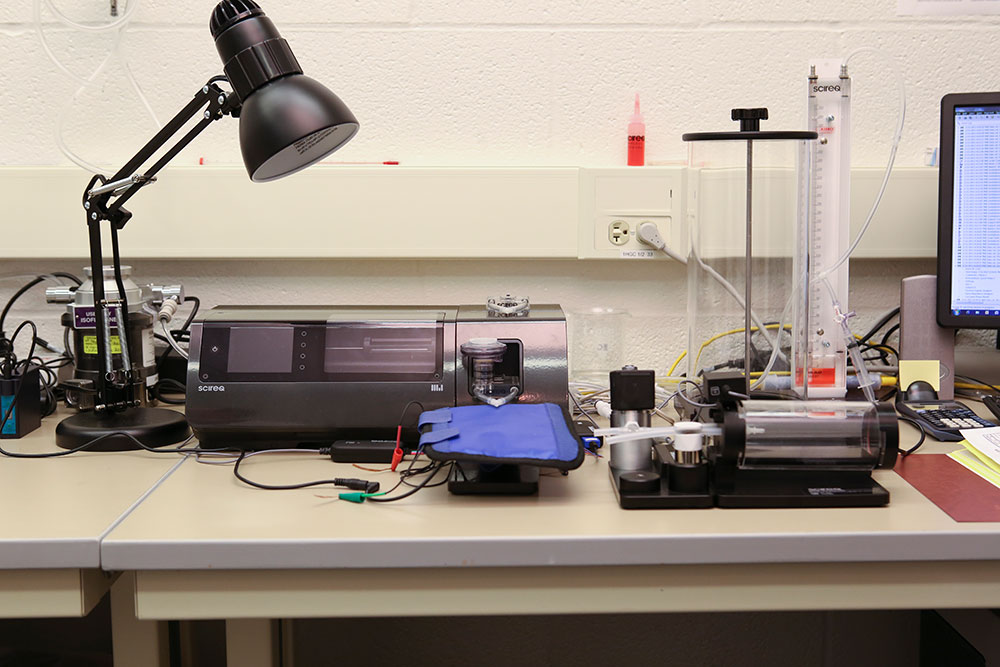
The SCIREQ flexiVent FX and other equipment are seen in the Pulmonary Immunology and Physiology Core at Penn State College of Medicine.
The flexiVent FX can be used for pre-clinical pulmonary research including the assessment of pulmonary function in rodent models including airway hyper-responsiveness, ventilation-induced lung injury, postnatal lung development, airway remodeling, chronic inflammation, allergy, environmental exposures (ozone, gases, particulates, smoke) and infection.
The instrument also has the capacity to deliver, measure and test the pre-clinical potential of therapeutic interventions via aerosol.
The inExpose is a compact integrated data acquisition and exposure system that can provide enhanced experimental control and monitoring for inhalation exposures.
Individually programmed servo-controlled pumps are used to produce repeated and reproducible exposure profiles and can be equipped with nebulizers to deliver both particulates and aerosols.
The nose-only configuration accommodates up to 12 subjects at once and reduces the quantity of materials needed for exposures.
The Data Sciences International FinePointe Whole Body Plethysmograph (WBP) system can be used to measure respiratory function in animals without the use of anesthesia or restraint.
The system relies on specially designed chambers in which subjects are placed and allowed to breathe under natural conditions. The chambers are fitted with pneumotachs screens that allow air to pass through with some resistance. The resistance created by these screens causes small pressure changes in the chambers relative to the ambient air that are measured by sensitive pressure transducers and from which flow can be derived.
When used in combination with the FinePointe software, the DSI FinePointe WBP system can be used to measure many physiologic and respiratory parameters including breaths-per-minute, tidal volume, minute volume, inspiratory time, expiratory time, pause and index of constriction.
The MouseOx Plus is the second generation of the original mouse pulse oximeter and physiological monitor. It is clinically validated and can be used to measure arterial oxygen saturation, heart rate, breath rate, temperature, pulse distention and breath distention on mice, rats and other small animals.
The OxyCycler A84X05 is a multi-chamber dynamic oxygen controller for researchers who do oxygen-sensitive work and makes complex oxygen profile control easy.
Using a PC interface, multiple, independent, controlled profiles can be created for different chambers with realtime trend charting, data logging and remote operation.
It can control oxygen profiles with multiple setpoints from 0.1 percent to 99.9 percent oxygen and can control both the time between setpoints and how many times the protocol repeats (one to 999, or loop infinitely).
The Nuaire NU-425-400 biological safety cabinet is a Class II, Type A2 cabinet that offers personnel, product and environmental protection to obtain optimum control over product quality while reducing the potential for exposure of both product and personnel to airborne biological or particulate chemical agents in low- to moderate-risk/hazard research and drug preparation or product operations.
flexiWare is data acquisition software that combines a powerful, flexible data flow control engine with an easy-to-use graphical user interface. flexiWare software allows users to operate SCIREQ hardware in an intuitive manner and focus on results, not equipment.
It provides support for several phases of the research cycle, including study planning, experimentation and data acquisition, as well as post-data collection review and reporting.
Data Sciences International FinePointe software is a powerful, easy-to-use tool for collecting, analyzing and reporting life science data.
Using special algorithms, FinePointe software utilizes outputs generated by the DSI FinePointe WBP to compute and analyze the data for many respiratory applications, including tidal parameters, indications of bronchoconstriction, indications of airway irritation and cough analysis. The software comes with a complete built-in reporting capability; however, all data can also be exported to Excel, text, OpenOffice and a variety of other statistical packages for report processing.
Procedures, Protocols and Forms
Standard Operating Procedure for working with Bio-Safety Level 2 (BSL-2) materials: Pulmonary Immunology and Physiology (PIP) Core
Director: E. Scott Halstead
Location: Lab CG737 – Biosafety Level 2
General precautions
- Anyone working with BSL2 materials who does not have documented vaccination against hepatitis B is offered this vaccination at no cost to them through Student or Employee Health as appropriate. Vaccination must be documented. If the person declines vaccination, this must be documented in writing through Student or Employee Health. Even if they initially decline vaccination, they can later decide to be vaccinated.
- Gowning must be done in the Anteroom of CG737 and is required before entering the Infection room (CG737A) to include lab coat, gloves, hairnet, surgical mask/respirator and shoe covers.
- No eating, drinking, smoking, handling contact lenses or applying cosmetics in the lab at any time.
- Mouth pipetting is prohibited; mechanical pipetting devices are to be used at all times.
- All work with BSL2 materials will be conducted within the Infection room and preferably within the biosafety cabinets (BSC) in Room CG737A. No work with BSL2 materials will be conducted in the Anteroom of CG737, which is reserved for gowning.
- All procedures are performed carefully to minimize the creation of splashes or aerosols.
- Lab doors are locked with limited access by key.
- Biohazard warning signs posted on the doors.
- Check the certification tag on the BSC to ensure the cabinet has been certified within the last 12 months. If it has not been certified within that time, notify the lab supervisor and they will arrange to have it certified. Do not do work in this BSC until it has been recertified.
- Turn on the blower in the BSC at least 10 minutes before placing human-derived materials in the cabinet.
- The BSC airflow (“Magnehelic”) gauge should be checked (reading is equal to approximately 0.5 inches) to assure proper operation of the cabinet before placing any materials into it. Readings indicate relative pressure drop across the HEPA filter. Higher readings may indicate filter clogging. Zero readings may indicate loss of filter integrity. In either case, notify lab supervisor to arrange to have the BSC tested.
- Gloves and lab coat must be worn at all times when manipulating human-derived materials. Eye protection is also recommended to avoid a splash exposure.
- Do not disrupt the airflow through the BSC by placing any item on the grills or by opening the door to the corridor.
- Do not use flames (e.g., Bunsen or alcohol burners) in BSC as this disrupts the appropriate airflow in the cabinet increasing the risk of accidental worker exposure. Heat from flames can also damage the HEPA filter of the BSC.
- Keep the number of items placed within the BSC to a minimum to avoid disrupting the airflow.
- In general, the interior of the hood should be considered to be a contaminated zone, even though every effort is made to keep the surfaces clean, as is consistent with accepted good microbiological practice and sterile technique.
- Work should be performed on the center of the work surface of the BSC whenever possible. Work outward progressing from clean to dirty (contaminated). Human-derived materials should not be placed directly adjacent to or directly on the intake grills.
- Minimize the number of times you move your hands in or out of the BSC. All movements disrupt the airflow and increase the risk of exposure to biohazards.
- All waste and disposable items are decontaminated within the BSC by wiping with a 2 percent bleach solution. Following decontamination, these items are disposed of by placing in biohazard trash bag or sharps container.
- Use of razor blades, scalpels, and hypodermic needles (“sharps”) should be kept to the absolute minimum. When use is absolutely unavoidable, these items are discarded into the sharps container in the BSC. Do not recap needles. Page pager number 1536 for pick-up and replacement of filled sharps containers.
- Keep a bottle of disinfectant 70 percent ethanol in the BSC for decontamination, or in case of a spill.
- After manipulating human-derived materials, tightly close all containers before removing them. Wipe down the surface of all equipment used in manipulations (pipettors, etc.) with 70 percent ethanol before removing from the cabinet.
- At the end of experimental work, clean the inside surfaces of the BSC by wiping the back and front grills, work surface, accessible walls and the inside of the window with 70 percent ethanol. A preliminary cleaning with detergent (e.g., diluted 7X laboratory detergent) is optional, but may be helpful for removing dirt or organic matter.
- Paper towels used to wipe down the hood are disposed of as biohazardous trash in red biohazard bags.
- Note 1: Bleach is harmful to stainless steel and should not be used unless it is rinsed off with water, followed by 70 percent ethanol.
- Note 2: Use of ultraviolet (UV) lights as the primary means to disinfect the BSC
is highly discouraged. UV lamps are rapidly covered by dust and dirt that hamper the appropriate emission of UV light. In addition, UV light does not kill infectious materials protected by dust, dirt or organic residue, and UV light does not penetrate cracks or through the grillwork of a BSC. UV rays from the BSC can harm individuals in the lab since they reflect off the stainless steel interior of the cabinet.
- Allow the blower to run for at least 10 minutes following use.
Exposure and transportation
Procedure to follow in the event of an accidental spill:
- When cleaning a spill, gloves and lab coat are worn at all times. Small spills on a surface such as a disposable blue lab pad should be treated with 70 percent ethanol and then disposed of in red biohazard bags. Red biohazard bags will be picked by environmental services and disposed of in the appropriate manner.
- In the event of a large spill, place a disposable covering over the spill, control access to the area of the spill, and call Environmental Health immediately (pager number 0889).
- Report the spill to Todd Umstead or Dr. E. Scott Halstead. See contact information here.
Procedure to follow in the event of an accidental exposure:
- In the event of an accidental exposure, wash the affected area thoroughly with soap and running water for 15 minutes. As appropriate, 70 percent ethanol can also be used to wash the affected area. Any mucous membranes exposed to materials should be flushed with copious amounts of water.
- Remove any clothing exposed to the materials as soon as reasonably possible. Contaminated clothing should be placed in a blue plastic laundry bag, labeled as soiled linen, and appropriate arrangements will be made for laundering. Gloves (e.g., vinyl or latex) will be used when handling clothing prior to washing.
- Wrap the wound.
- If between 7 a.m. and 5 p.m. Monday through Friday, go to Employee Health, Room H1507, close to the Hospital’s North Entrance. During other times, go to the Emergency Room. Any individual who incurs an exposure incident will be offered post-exposure evaluation and followup in accordance with the OSHA standard and PA Act 148. Post exposure prophylaxis in accordance with the current recommendations of the U.S. Public Health Service will be provided. In addition, appropriate counseling concerning precautions to take during the period after the exposure incident will be given. Employee Health or the Emergency Department will also provide information on what potential illnesses to be alert for and will report any related experiences to appropriate personnel.
- Report the spill to Todd Umstead or Dr. E. Scott Halstead (see contact information here) as well as to the Biosafety Officer, currently Ray Scheetz, at 717-531-5573 or biosafetyofficer@pennstatehealth.psu.edu.
- All human blood and tracheal aspirate secretions will be transported from the patient care area using double-sealed, shatterproof containers. Gloves will not be worn during transport.
Changes
This SOP was developed by Jill Raymond on Feb. 9, 2009, and updated by E. Scott Halstead on July 1, 2015.
The template this SOP is based on was developed by Penn State Health’s Biosafety and Recombinant DNA Committee on Jan. 23, 2009, by modification of template protocols from the Arizona State University Standard Operating Procedures Manual and the Wake Forest University School of Medicine Biosafety Standard Operating Procedures Template.
- Evaluation of Respiratory System Mechanics in Mice using the Forced Oscillation Technique (McGovern, T.K., Robichaud, A., Fereydoonzad, L., Schuessler, T. F., Martin, J.G. J. Vis. Exp. (75), e50172, doi:10.3791/50172 (2013)
- See Penn State Health Milton S. Hershey Medical Center Infection Prevention and Control Plan in the Policy Portal (ePass login required)
- See Penn State Health Milton S. Hershey Medical Center Bloodborne Pathogens Safety Program in the Policy Portal (ePass login required)
- See sample protocol snapshot form in OneDrive (Penn State Access ID login required)
Work With Pulmonary Immunology and Physiology
Fees are determined and approved by the Controllers Office to cover actual costs of providing the core’s services.
Contact the core for current pricing.
The schedule for the Pulmonary Immunology and Physiology Core is available in CalendarHost.
