The electronic Internal Approval Form (IAF) is a required internal form used at the College of Medicine to provide a summary of pertinent administrative and fiscal information about a proposed sponsored project. The IAF is initiated by the department administrator after the SIMS budget is created.
Jump to topic
Search
IAF Training Guide for Basic Science Agreements
The Internal Approval Form (IAF) is designed to ensure proper review and vetting of any proposed study.
Things to Know
All required fields in the IAF are marked with an asterisk. The IAF cannot be submitted into workflow if any required field is incomplete.
Any fields that are pale yellow need to be filled in. All fields must be green.
If unsure of what to enter in a particular field, hovering the mouse over the ? icon will show a balloon providing greater detail about the requirement. Many fields also include examples to further assist.
All uploaded attachments must be in Adobe PDF format.
The home screen is the first screen seen upon logging in to IAF.
Search for existing IAFs using the “OSP Number” search box at the upper right of the screen.
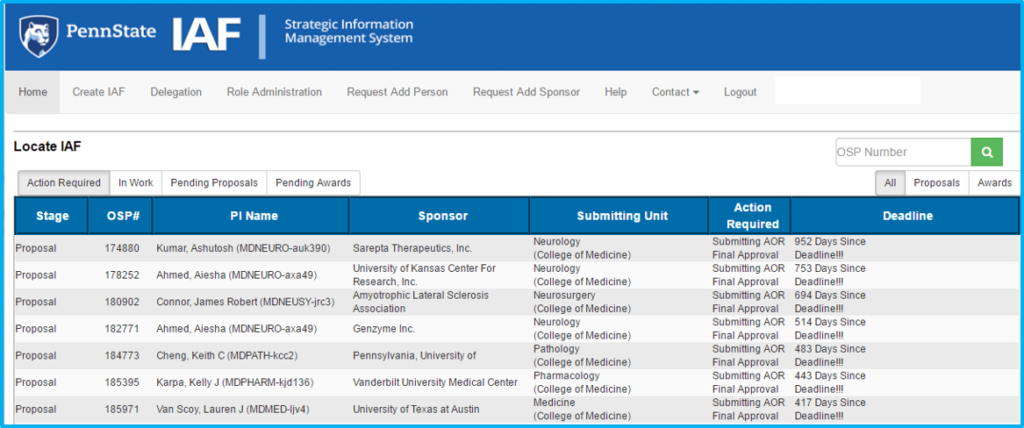
To create a new IAF, click “Create IAF” in the top menu.
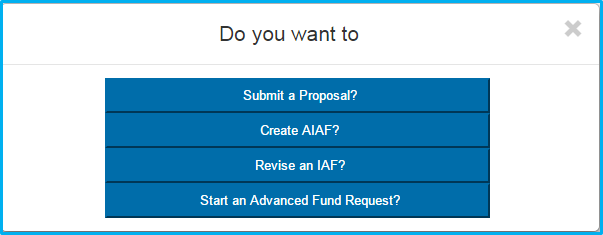
Users will be prompted with four potential selections:
- Submit a Proposal
- Create AIAF
- Revise an IAF
- Start an Advanced Fund Request
This training guide focuses on the most commonly used, “Submit a Proposal.” Click that to proceed.
After clicking “Submit a Proposal,” users will be prompted with five possible selections:
- New Funds
- A Supplement to an Existing Award
- A Competitive Renewal
- A Non-Competing Continuation
- Resubmission of a Prior Proposal
This training guide focuses on the most commonly used, “New Funds.” Click that to proceed.

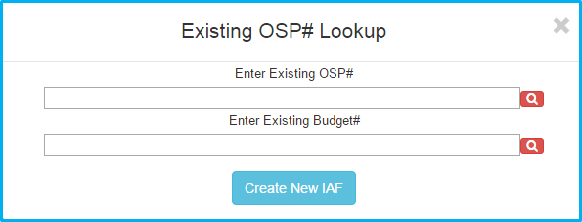
A screen appears that give choices for looking up a project by existing OSP number, as well as a “Create New IAF” button.
Click the “Create New IAF” button to proceed.
Note: Users are not required to pull in information from either an existing OSP or budget, unless this is an NIH clinical trials subaward continuation. If continuation, it will need to be indicated as such on the “Basic Info” tab.
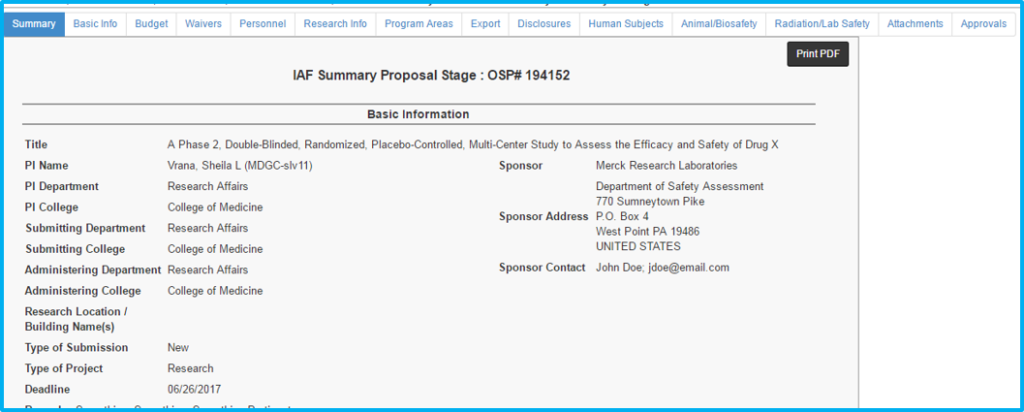
The IAF Summary Tab provides a read-only summary of all information entered on the individual tabs.
Note: When initially creating a new IAF, users will skip this tab and be directed to the “Basic Info” tab to begin entering information.
Program Title
Do not include an investigational compound or device name in the title. Change to something generic, such as Compound X, Device A, etc.
The first letter of each word should be capitalized with the exception of articles (a, in, an, the, etc.). The remaining letters should be lowercase. An example is seen in the screenshot below – “Laboratory Testing of Compound X in Cell Systems.”
Principal Investigator (PI)
Type in the PI last name and choose from the dropdown list to verify. IAF will not allow entry unless the name is verified from the dropdown list.
Before verifying the name, a magnifying glass search icon will appear with a red background.
After verifying the name, a checkmark icon will appear with a green background.
Sponsor
Begin typing the sponsor name, choose the appropriate one from the dropdown list and click the magnifying glass to verify. IAF will not allow entry unless the name is verified from the dropdown list.
If a sponsor has multiple addresses, be sure to select the appropriate one. Click the magnifying glass icon to the right of the Sponsor Address field and select the appropriate one from the dropdown list in order to validate.
If a contract is between Penn State and a Clinical Research Organization (CRO), list the sponsor, not the CRO, in the “Sponsor Name” field. List the CRO in the “Remarks” field.
If unable to locate the sponsor in the system, they may need to be added. Contact ORA for assistance.
In the “Sponsor Contact” field, list a point of contact including name and email address to assist in contract negotiation.
Type of Submission
For basic science agreements, select “New.” Some exceptions apply, but this is the most commmon.
Type of Project
Select “Research.”
![]()
Deadline Date
For basic science, the date must be at least two months from the current date.
Remarks
Add pertinent notes, such as CRO name or PSCI number, to this box.
Potential Issue
If there is a yellow field for “Please Enter Approval Date for the following: Sponsor Issues,” leave that field blank.
Budget Details with Salary Information
Upload the SIMS budget (if available) or other budget documentation on the “Attachments” tab.
Budget
Start: Choose an estimated start date.
End: Choose an estimated end date based on anticipated length of the study. This must be the last day of the month that is selected.
Information entered in “Initial Period” will auto-populate in “Total Budget.”
Note: Use proposal estimates. When contract/budget negotiations are complete, the Statement of Award will reflect the actuals.
F&A Rate
F&A: Indirect/Overhead
F&A is calculated by multiplying the “Direct” amount by the F&A rate.
For basic science agreements, F&A rate will vary by sponsor:
- For government/nonprofits, rate is current full rate.
- For industry-sponsored rate, add 5 percent to the current full rate.
- For other sponsors, see the “Waiver” Tab.
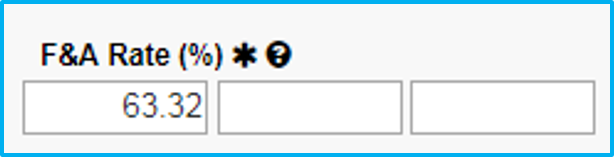
The image below shows the F&A Rate field with a sample rate of 63.32 filled in. This is for purposes of illustration only. The actual F&A rate to be used varies, as it is reviewed and approved annually and is subject to change.
The University offers information on F&A, including a copy of the current rate agreement, here.
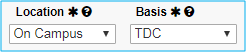
Location and Basis
Location will always be “On Campus.”
For Basis, select “TDC” (total direct costs).
Add SIMBA Account Information
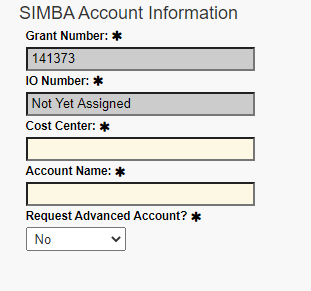
Under SIMBA Account Information, fill in the Cost Center. Contact the appropriate financial analyst for assistance.
Under “Request Advanced Account?,” select Yes or No.
Note that the Grant Number is auto-populated, along with the Account Name field. IO Number will say “Not Yet Assigned.”
For type of F&A, choose full or partial.
If Choosing Full
- No waiver is required.
- Whatever rate it was awarded at carries through for all years of the agreement.
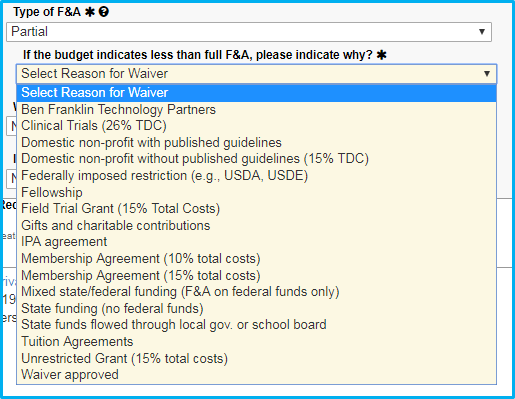
If the reason for the partial F&A rate is a “Domestic non-profit with published guidelines,” upload a copy of the guidelines.
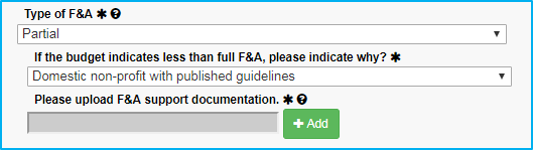
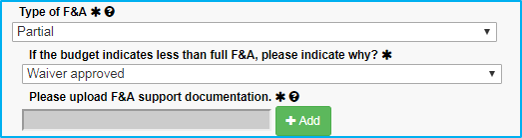
If the reason for the waiver is not in the dropdown list, choose the final selection, “Waiver approved.” When doing this:
- Approval must be obtained from Dr. Sheila Vrana.
- Dr. Vrana’s approval must be saved as a PDF file and attached.
- The submission must include the reason why the waiver is approved or reference a published NIH guideline.
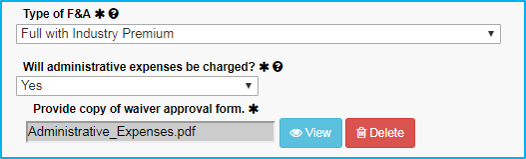
Administrative Expenses
Administrative expenses (such as office supplies and clerical supplies) may not be directly charged when federal funds are used, except in special circumstances.
The University offers additional details regarding such F&A exceptions.
Requests for exceptions can be submitted using the University’s Cost Accounting Justification Form.
If “Will administrative expenses be charged?” is set to Yes, ensure that an approval email or document is attached in the Attachments tab.
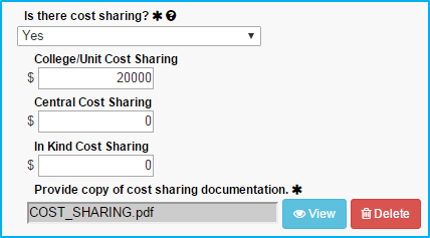
Cost-Sharing
In the event the sponsor is unwilling to cover the full costs of the study, resulting in a deficit, cost-sharing will be needed to move the study forward.
The University offers more information on cost-sharing.
All cost-sharing must be approved by the applicable department chair in writing. Investigators may not know up front whether cost-sharing is needed. If “No” is selected initially, it can be corrected at a later time when the need is identified.
If “Yes” is selected for cost-sharing, ensure that an approval document is attached in the Attachments tab.
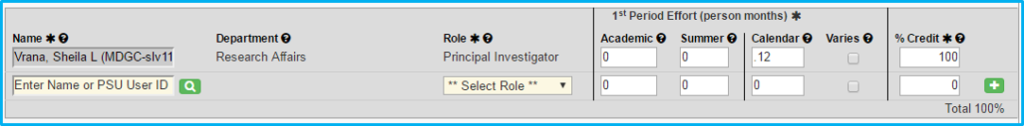
Personnel
List all study personnel, including principal investigator, co-investigator, coordinator, etc.
Effort
Complete for principal investigator.
- For industry-sponsored, if 1 percent, then enter .12 for PI. If 2 percent, then enter .24.
- For federally funded, enter what it actually is (e.g., 10 months’ effort would be 1.2).
Use this person-month calculator for assistance.
Credit
Credit is only awarded to PIs and co-PIs.
The “% Credit” column must total 100 percent.
If each co-investigator has a 1 entered, then the PI will get the balance to equal 100.
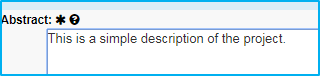
Abstract
Enter a simple description of the project in this field.
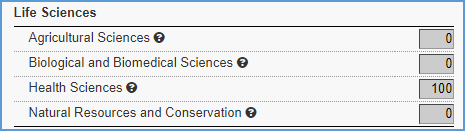
Scientific Areas
For basic science agreements, allocate 100 percent in either the “Health Sciences” or the “Biological and Biomedical Sciences” field under Life Sciences.
Consult with the PI, coinvestigator(s), study coordinator and/or department research administrator to determine which program areas are applicable to the study.
Check all applicable areas.

The second question on the Export tab will be auto-populated based upon the name/address entered for the sponsor.
Each question must be answered accurately. If in doubt, check with the PI or other personnel (coordinator, sponsor contact if applicable, etc.) Don’t guess.

This is completed by investigators and coordinators as part of the workflow process.
On the Waiver tab, if either “Unrestricted grant” or “Gifts and charitable contributions” is selected, then the investigator and key personnel will be asked three additional questions when completing the Disclosures tab.

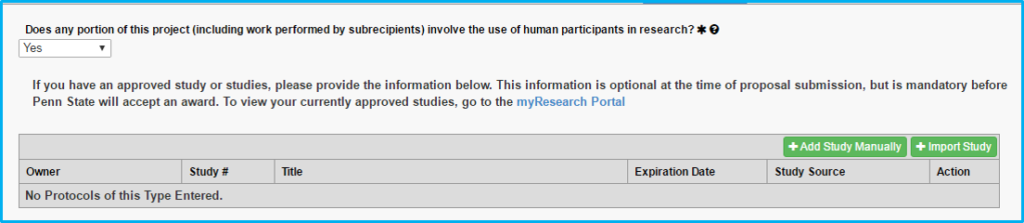
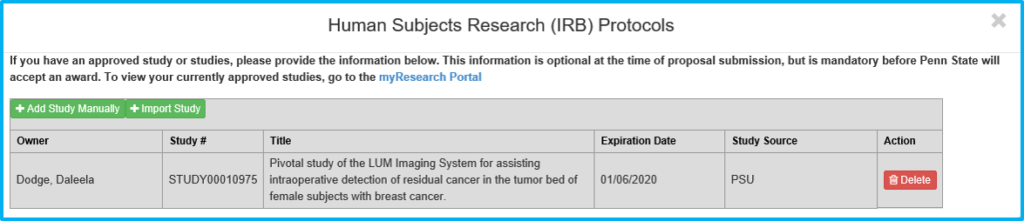
If “Yes” is chosen for “Does any portion of the project involve the use of human participants in research?” use the Import Study button to link to and import the IRB information. This is done after IRB approval. Do not import pending approvals.
Protocol on this tab actually means the IRB approval.
The grey box will not contain any information until the study is imported following IRB approval.
Workflow can be started without IRB approval. However, IRB approval is needed prior to award.
Please note that studies determined by the IRB to be human subjects exempt and studies being managed by an external IRB will not have an expiration date.
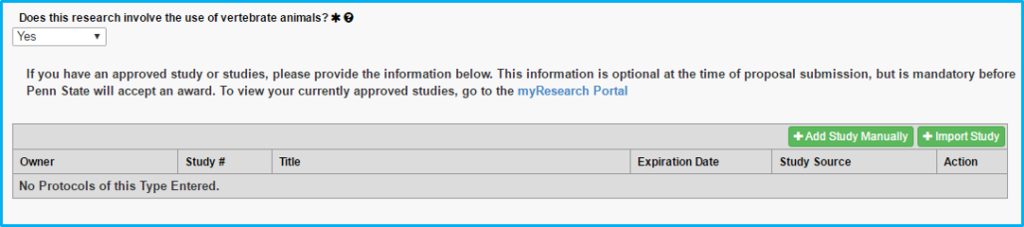
Vertebrate Animals
If choosing “yes” to whether vertebrate animals are used, a current IACUC approval is required.
A box will appear allowing the import of the IACUC approval. “Protocol” on this tab actually means the IACUC approval.
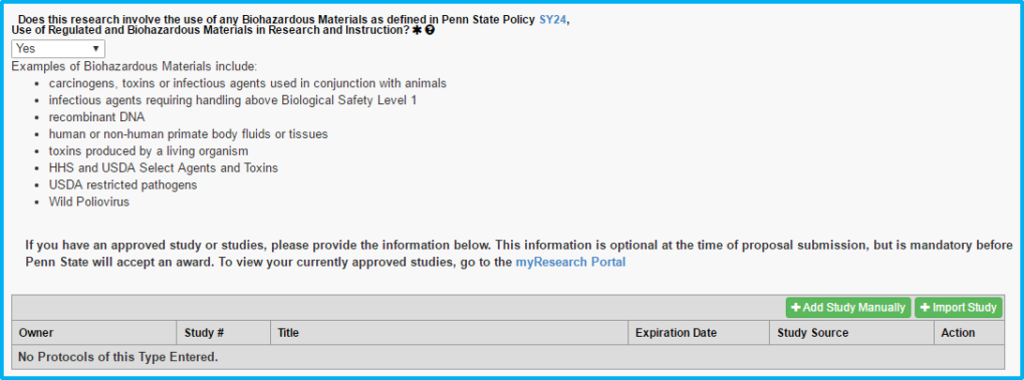
Biohazardous Materials
If choosing “yes” to whether biohazardous materials are used, a current biohazard safety approval is required.
The import study feature will not work for biosafety approval. The information must be added manually.
“Protocol” on this tab actually means the biosafety approval.
“Study #” is not the CATS IRB number, but instead is a unique number assigned by the Institutional Biosafety Committee (IBC).
The approval letter (e-mail) from the IBC will contain this unique number along with an expiration date.
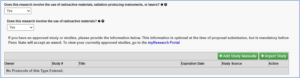
This tab initially shows two questions, but selecting “Yes” to the first question will result in additional selections.

Please reach out to the Radiation Safety Committee (RSC) for all questions involving radioactive materials, radiation producing instruments, or lasers by contacting Heather Barber via hhb104@psu.edu or Brian Lorah via bjl14@psu.edu.
If selecting “Yes” to the question, “Does the research involve the use of radioactive materials, radiation-producing instruments, or lasers?”, you will be prompted with three additional questions.

If selecting “Yes” that radioactive materials are used, then a current radiation approval is required.
The “Import Study” feature will not work for radiation approval. This information must be added manually.
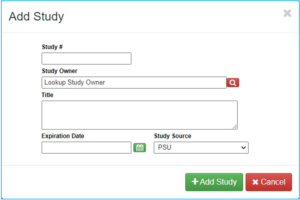
“Study #” is not the CATS IRB number, but instead is a unique number assigned by Radiation Safety Committee (RSC).
“Study Owner” is the investigator who received approval from the RSC to manage the radioactive materials. This may be an investigator other than the PI.
“Title” will be either the general approval name for the investigator approved to manage the radioactive materials or a project specific title.
There will be an expiration date given and this needs to be placed into the IAF.
Warning Message
A warning message that appears at the top of the “Attachments” Tab about not uploading award documents should be ignored. Those instructions are applicable to projects at University Park only, not to the College of Medicine.

What to Attach
- For “Budget Documentation With Salary Information,” upload the budget worksheet.
- For “Full Proposal Upload,” upload the protocol or scope of work.
- Under “Add Other Proposal Document,” include any cost-sharing approval document, if applicable, and choose the appropriate type and enter a description.
- Under “Add Other Proposal Document,” include any Confidential Disclosure Agreement (CDA), if applicable, and choose the appropriate type and enter a description.
Maximum document size is 100MB.
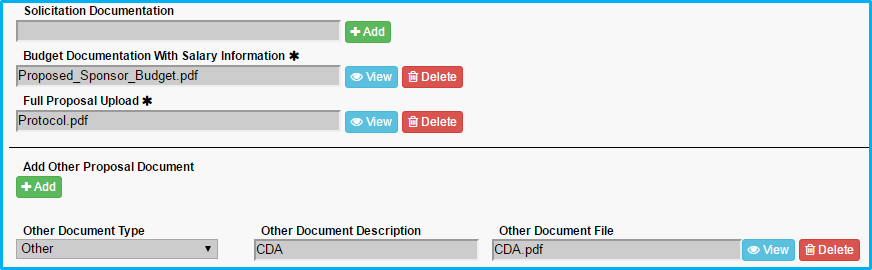
Verify all required fields/questions have been completed by clicking on the “Summary” tab.
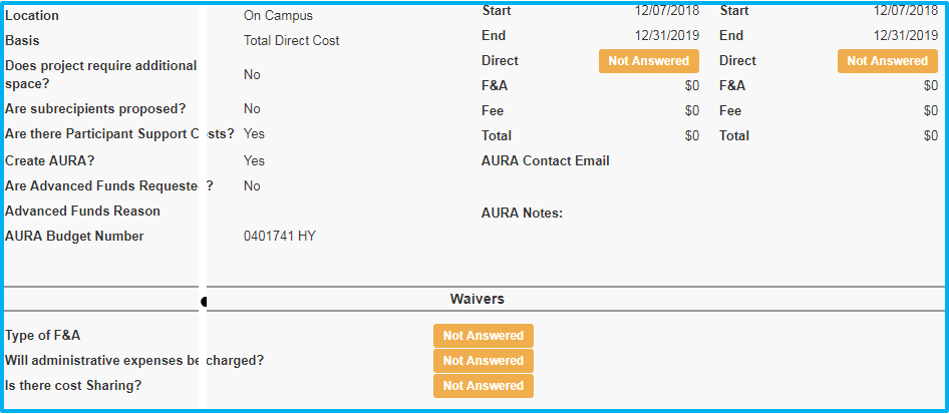
Any required field that is not complete will include a “Not Answered” box with a yellow background.
If an investigator (including the primary investigator) is removed after the workflow has been started, it will be noted on this tab.
Once the IAF is a “pending proposal,” the investigator’s and key personnel’s responses to the Disclosure tab will be visible on the summary.
The workflow should be started prior to submitting a Basic Science Agreement Request.
After approving an IAF, the user will be redirected to the IAF Approval Confirmation Page.
Once approved by the PI, the approvals are workflowed to any co-investigators simultaneously.
IAF training is available. Email e-grants@pennstatehealth.psu.edu or e-contracts@pennstatehealth.psu.edu as appropriate for details or to schedule.
Also, please ensure anyone who is listed on an IAF has their Penn State (@psu.edu) email forwarded to their Penn State Health (@pennstatehealth.psu.edu) email account so they receive the IAF alerts. See instructions for setting up email forwarding.
IAF Training Guide for Clinical Trials Agreements
The Internal Approval Form (IAF) is designed to ensure proper review and vetting of any proposed study.
Things to Know
All required fields in the IAF are marked with an asterisk. The IAF cannot be submitted into workflow if any required field is incomplete.
Any fields that are pale yellow need to be filled in. All fields must be green.
If unsure of what to enter in a particular field, hovering the mouse over the ? icon will show a balloon providing greater detail about the requirement. Many fields also include examples to further assist.
All uploaded attachments must be in Adobe PDF format.
The home screen is the first screen seen upon logging in to IAF.
Search for existing IAFs using the “OSP Number” search box at the upper right of the screen.

To create a new IAF, click “Create IAF” in the top menu.

Users will be prompted with four potential selections:
- Submit a Proposal
- Create AIAF
- Revise an IAF
- Start an Advanced Fund Request
This training guide focuses on the most commonly used, “Submit a Proposal.” Click that to proceed.

After clicking “Submit a Proposal,” users will be prompted with five possible selections:
- New Funds
- A Supplement to an Existing Award
- A Competitive Renewal
- A Non-Competing Continuation
- Resubmission of a Prior Proposal
This training guide focuses on the most commonly used, “New Funds.” Click that to proceed.

A screen appears that give choices for looking up a project by existing OSP number, as well as a “Create New IAF” button.
Click the “Create New IAF” button to proceed.

Note: Users are not required to pull in information from either an existing OSP or budget, unless this is an NIH clinical trials subaward continuation. If continuation, it will need to be indicated as such on the “Basic Info” tab.
The IAF Summary Tab provides a read-only summary of all information entered on the individual tabs.
Note: When initially creating a new IAF, users will skip this tab and be directed to the “Basic Info” tab to begin entering information.

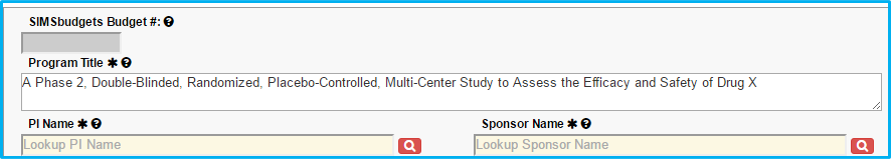
Program Title
Do not include an investigational drug or device name in the title. Change to something generic, such as Drug A, Device X, etc.
The first letter of each word should be capitalized with the exception of articles (a, in, an, the, etc.). The remaining letters should be lowercase. An example is seen in the screenshot below – “A Phase 2, Double-Blinded, Randomized, Placebo-Controlled, Multi-Center Study to Assess the Efficacy and Safety of Drug X.”
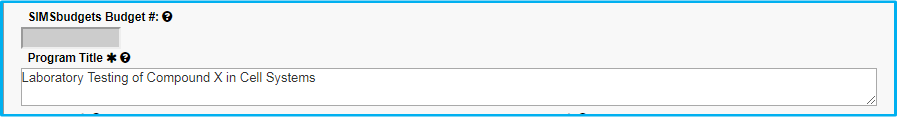
Principal Investigator (PI)
Type in the PI last name and choose from the dropdown list to verify. IAF will not allow entry unless the name is verified from the dropdown list.
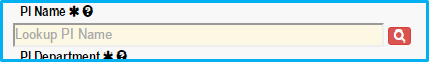
Before verifying the name, a magnifying glass search icon will appear with a red background.

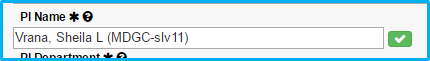
After verifying the name, a checkmark icon will appear with a green background.

Sponsor
Begin typing the sponsor name, choose the appropriate one from the dropdown list and click the magnifying glass to verify. IAF will not allow entry unless the name is verified from the dropdown list.
Note: After typing the first three letters of the sponsor name, after a pause, a list of any applicable sponsors will be displayed.

If a sponsor has multiple addresses, be sure to select the appropriate one. Click the magnifying glass icon to the right of the Sponsor Address field and select the appropriate one from the dropdown list in order to validate.
If a contract is between Penn State and a Clinical Research Organization (CRO), list the sponsor, not the CRO, in the “Sponsor Name” field. List the CRO in the “Remarks” field.
If unable to locate the sponsor in the system, they may need to be added. Contact ORA for assistance.
In the “Sponsor Contact” field, list a point of contact including name and email address to assist in contract negotiation.

Type of Submission
For clinical trials agreements, select “New.” Some exceptions apply, but this is the most commmon.

Type of Project
If human subjects are involved, select “Clinical Trial.” If human subjects are not involved, select “Research.”
![]()
Deadline Date
For industry-sponsored clinical trials, the date must be at least three months from the current date.

Remarks
Add pertinent notes, such as CRO name or PSCI number, to this box.

Potential Issue
If there is a yellow field for “Please Enter Approval Date for the following: Sponsor Issues,” leave that field blank.
After completing all required fields on the “Basic Info” tab, click the “Save” button on the bottom right before moving on to the remaining tabs.
This will result in creation of the draft IAF including a unique OSP number, which appears on the top left portion of the screen and should be recorded for the submitter’s records.

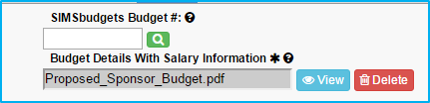
Budget Details with Salary Information
Upload the proposed sponsor budget (estimated budget) on the “Attachments” tab.

Budget
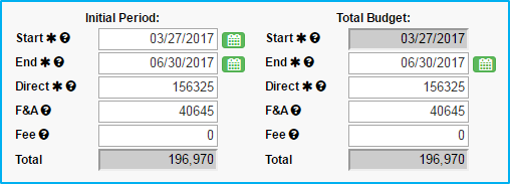
Start: Choose an estimated start date.
End: Choose an estimated end date based on anticipated length of the study. This must be the last day of the month that is selected.
Information entered in “Initial Period” will auto-populate in “Total Budget.”
Note: Use proposal estimates. When contract/budget negotiations are complete, the Statement of Award will reflect the actuals.

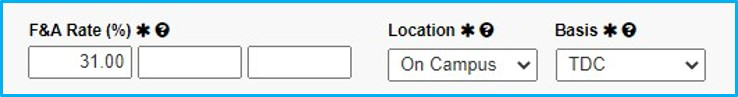
F&A Rate
For industry-sponsored clinical Trials, F&A rate will be 31%, unless there is a waiver; then, see the “Waiver” Tab.
F&A: Indirect/Overhead
F&A is calculated by multiplying the “Direct” amount by the F&A rate.
Location and Basis
Location will always be “On Campus.”
For Basis, select “TDC” (total direct costs) for industry-sponsored clinical trials.
For federally funded, choose “MTDC” (modified total direct costs).
Add SIMBA Account Information
Under SIMBA Account Information, fill in the Cost Center. Contact the appropriate financial analyst for assistance.
Under “Request Advanced Account?,” select Yes or No.
Note that the Grant Number is auto-populated, along with the Account Name field. IO Number will say “Not Yet Assigned.”

For type of F&A, choose partial, then choose
“Clinical Trial Sponsored by Industry.”
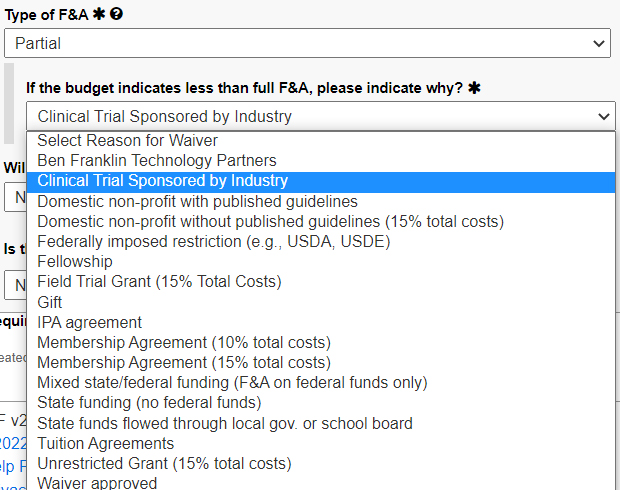
If less than 31 percent, a wavier is required.
If the reason for the waiver is a “Domestic non-profit with published guidelines,” upload a copy of the guidelines.

If the reason for the waiver is not in the dropdown list, choose the final selection, “Waiver approved.” When doing this:
- Approval must be obtained from Dr. Sheila Vrana.
- Dr. Vrana’s approval must be saved as a PDF file and attached.
- The submission must include the reason why the waiver is approved or reference a published NIH guideline.

Administrative Expenses
Administrative expenses (such as office supplies and clerical supplies) may not be directly charged when federal funds are used, except in special circumstances.
The University offers additional details regarding such F&A exceptions.
Requests for exceptions can be submitted using the University Cost Accounting Justification Forms.
If administrative expenses will be charged answer “Yes” and ensure that an approval email or document is attached in the “Attachments” tab.
Cost-Sharing
In the event the sponsor is unwilling to cover the full costs of the study, resulting in a deficit, cost-sharing will be needed to move the study forward.
The University offers more information on cost-sharing.
All cost-sharing must be approved by the applicable department chair in writing. Investigators may not know up front whether cost-sharing is needed. If “No” is selected initially, it can be corrected at a later time when the need is identified.
If “Yes” is selected for cost-sharing, ensure that an approval document is attached in the Attachments tab.

Personnel
List all study personnel, including principal investigator, co-investigator, coordinator, etc.
Effort
Complete for principal investigator.
- For industry-sponsored, if 1 percent, then enter .12 for PI. If 2 percent, then enter .24.
- For federally funded, enter what it actually is (e.g., 10 months’ effort would be 1.2).
Use this person-month calculator for assistance.
Credit
Credit is only awarded to PIs and co-PIs.
The “% Credit” column must total 100 percent.
If each co-investigator has a 1 entered, then the PI will get the balance to equal 100.

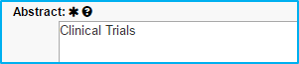
Abstract
“Clinical Trials” should be typed in the box for industry-sponsored clinical trials only.
Scientific Areas
For clinical trials, allocate 100 percent in the “Health Sciences” field under Life Sciences.

Consult with the PI, coinvestigator(s), study coordinator and/or department research administrator to determine which program areas are applicable to the study.
Check all applicable areas.

The second question on the Export tab will be auto-populated based upon the name/address entered for the sponsor.
Each question must be answered accurately. If in doubt, check with the PI or other personnel (coordinator, sponsor contact if applicable, etc.) Don’t guess.

This is completed by investigators and coordinators as part of the workflow process.

If “Yes” is chosen for “Does any portion of the project involve the use of human participants in research?” use the Import Study button to link to and import the IRB information. This is done after IRB approval. Do not import pending approvals.
Protocol on this tab actually means the IRB approval.
The grey box will not contain any information until the study is imported following IRB approval.
Workflow can be started without IRB approval. However, IRB approval is needed prior to award.
Please note that studies determined by the IRB to be human subjects exempt and studies being managed by an external IRB will not have an expiration date.

Vertebrate Animals
If choosing “yes” to whether vertebrate animals are used, a current IACUC approval is required.
A box will appear allowing the import of the IACUC approval. “Protocol” on this tab actually means the IACUC approval.

Biohazardous Materials
If choosing “yes” to whether biohazardous materials are used, a current biohazard safety approval is required.
The import study feature will not work for biosafety approval. The information must be added manually.
“Protocol” on this tab actually means the biosafety approval.
“Study #” is not the CATS IRB number, but instead is a unique number assigned by the Institutional Biosafety Committee (IBC).
The approval letter (e-mail) from the IBC will contain this unique number along with an expiration date.

This tab initially shows two questions, but selecting “Yes” to the first question will result in additional selections.

Please reach out to the Radiation Safety Committee (RSC) for all questions involving radioactive materials, radiation producing instruments, or lasers by contacting Heather Barber via hhb104@psu.edu or Brian Lorah via bjl14@psu.edu.
If selecting “Yes” to the question, “Does the research involve the use of radioactive materials, radiation-producing instruments, or lasers?”, you will be prompted with three additional questions.

If selecting “Yes” that radioactive materials are used, then a current radiation approval is required.
The “Import Study” feature will not work for radiation approval. This information must be added manually.


“Study #” is not the CATS IRB number, but instead is a unique number assigned by Radiation Safety Committee (RSC).
“Study Owner” is the investigator who received approval from the RSC to manage the radioactive materials. This may be an investigator other than the PI.
“Title” will be either the general approval name for the investigator approved to manage the radioactive materials or a project specific title.
There will be an expiration date given and this needs to be placed into the IAF.
Warning Message
A warning message that appears at the top of the “Attachments” Tab about not uploading award documents should be ignored. Those instructions are applicable to projects at University Park only, not to the College of Medicine.

What to Attach
- For “Budget Documentation With Salary Information,” upload the budget worksheet.
- For “Full Proposal Upload,” upload the protocol or scope of work.
- Under “Add Other Proposal Document,” include any cost-sharing approval document, if applicable, and choose the appropriate type and enter a description.
- Under “Add Other Proposal Document,” include any Confidential Disclosure Agreement (CDA), if applicable, and choose the appropriate type and enter a description.
Maximum document size is 100MB.

Verify all required fields/questions have been completed by clicking on the “Summary” tab.
Any required field that is not complete will include a “Not Answered” box with a yellow background.
If an investigator (including the primary investigator) is removed after the workflow has been started, it will be noted on this tab.
Once the IAF is a “pending proposal,” the investigator’s and key personnel’s responses to the Disclosure tab will be visible on the summary.

The workflow should be started as soon as possible after IAF creation to avoid potential delays in the contract award process.
After approving an IAF, the user will be redirected to the IAF Approval Confirmation Page.
Once approved by the PI, the approvals are workflowed to any co-investigators simultaneously.

At some point after the IAF is in workflow, it will need to be updated to include any applicable compliance approvals, such as from Human Subjects Protection Office, IACUC or Biosafety.
Previously this required recalling the IAF from workflow so it could be edited. However, this is no longer the case.
Note: Do not import pending compliance approvals. The compliance approval must be final in order to import the applicable expiration date.
To add a compliance approval, click “Create IAF” in the top left, as if creating a new IAF.
Then select “Add/Delete Study Number to IAF.”
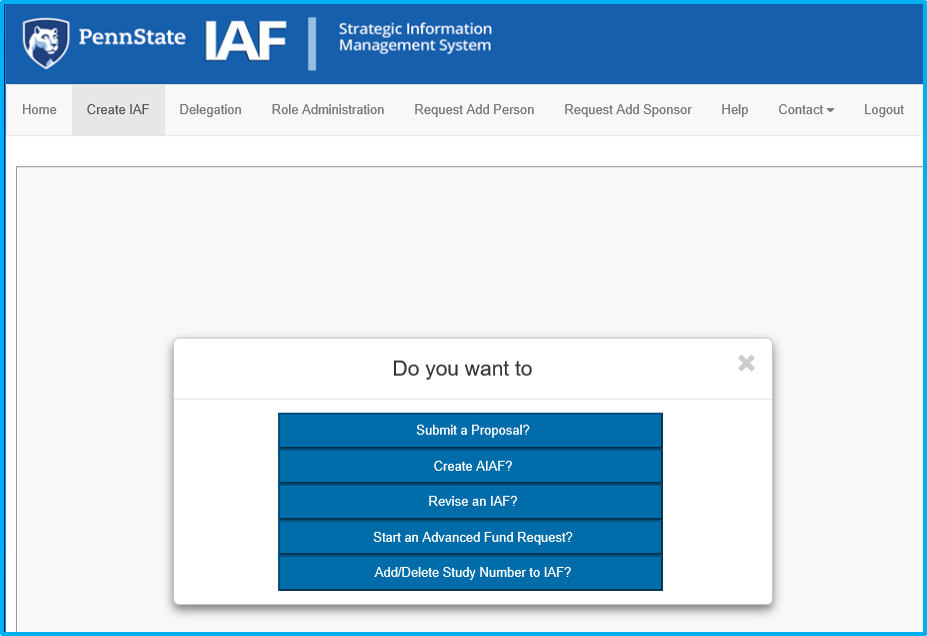
A prompt requesting the OSP number will appear. Type the applicable OSP number, then click on the red magnifying glass icon or hit enter on the keyboard to validate.
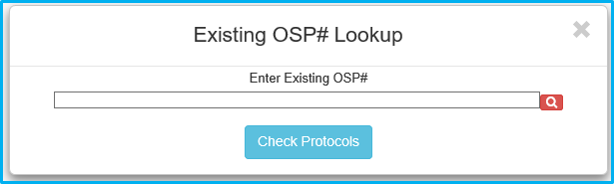
Once validated, the magnifying glass will turn into a green checkmark. Click “Check Protocols” to continue.
The “Available Protocol Type Selection” prompt will appear. The list will vary based upon the compliance approvals applicable to the IAF.

Click the desired compliance approval to continue.
This will bring up the applicable compliance approval add/import box. Click “Import Study(s)” and a list of studies will appear.
Click the checkbox beside the applicable study and then click “Import Study(s).”

This will then redirect back to the applicable compliance approval add/import box, which now includes the imported approval.
Go back into the IAF and click on the applicable tab to verify the compliance approval has been successfully imported.
IAF training is available. Email e-grants@pennstatehealth.psu.edu or e-contracts@pennstatehealth.psu.edu as appropriate for details or to schedule.
Also, please ensure anyone who is listed on an IAF has their Penn State (@psu.edu) email forwarded to their Penn State Health (@pennstatehealth.psu.edu) email account so they receive the IAF alerts. See instructions for setting up email forwarding.
IAF Training Guide for Grants
The Internal Approval Form (IAF) is designed to ensure proper review and vetting of any proposed study.
Things to Know
All required fields in the IAF are marked with an asterisk. The IAF cannot be submitted into workflow if any required field is incomplete.
Any fields that are pale yellow need to be filled in. All fields must be green.
If unsure of what to enter in a particular field, hovering the mouse over the ? icon will show a balloon providing greater detail about the requirement. Many fields also include examples to further assist.
All uploaded attachments must be in Adobe PDF format.
The home screen is the first screen seen upon logging in to IAF.
Search for existing IAFs using the “OSP Number” search box at the upper right of the screen.

To create a new IAF, click “Create IAF” in the top menu.

Users will be prompted with four potential selections:
- Submit a Proposal
- Create AIAF
- Revise an IAF
- Start an Advanced Fund Request
This training guide focuses on the most commonly used, “Submit a Proposal.” Click that to proceed.

After clicking “Submit a Proposal,” users will be prompted with five possible selections:
- New Funds
- A Supplement to an Existing Award
- A Competitive Renewal
- A Non-Competing Continuation
- Resubmission of a Prior Proposal
This training guide focuses on the most commonly used, “New Funds.” Click that to proceed.

A screen appears that give choices for looking up a project by existing OSP number, as well as a “Create New IAF” button.
Enter SIMS budget number in “Existing Budget #” and click the red magnifying glass to the right.
After the magnifying glass turns green, click the “Create New IAF” button to proceed.

The IAF Summary Tab provides a read-only summary of all information entered on the individual tabs.
Note: When initially creating a new IAF, users will skip this tab and be directed to the “Basic Info” tab to begin entering information.

Program Title
This information will prepopulate from the SIMS budget.
Principal Investigator (PI)
This information will prepopulate from the SIMS budget.
Sponsor
This information will prepopulate from the SIMS budget.
If a sponsor has multiple addresses, be sure to select the appropriate one. Click the magnifying glass icon to the right of the Sponsor Address field and select the appropriate one from the dropdown list in order to validate.
Type of Submission
This information should prepopulate.
Type of Project
This information should prepopulate. In most cases, the answer will be “Research.” If the project is a clinical trial, select “Clinical Trial” here.
![]()
Deadline Date
Enter sponsor deadline.

Remarks
Add pertinent notes to this box. This is optional.

Budget Details with Salary Information
Upload the SIMS budget (if available) or other budget documentation on the “Attachments” tab.

Budget
This information will prepopulate from SIMS.
Information entered in “Initial Period” will auto-populate in “Total Budget.”
F&A Rate
This information will prepopulate from SIMS.
The University offers information on F&A, including a copy of the current rate agreement, here.
Location and Basis
Location will always be “On Campus.”
For Basis, select “MTDC” for federal sponsors, such as NIH. Select “TDC” for non-federal sponsors.
Add SIMBA Account Information
Under SIMBA Account Information, fill in the Cost Center. Contact the appropriate financial analyst for assistance.
Under “Request Advanced Account?,” select Yes or No.
Note that the Grant Number is auto-populated, along with the Account Name field. IO Number will say “Not Yet Assigned.”

For type of F&A, choose full or partial.
If Choosing Full
- No waiver is required.
- Whatever rate it was awarded at carries through for all years of the agreement.
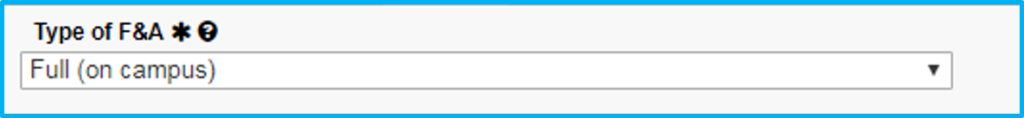
If Choosing Partial
If not charging the full F&A rate, indicate why by choosing the appropriate selection from the dropdown.

If the reason for the partial F&A rate is a “Domestic non-profit with published guidelines,” upload a copy of the guidelines.

If the reason for the waiver is not in the dropdown list, choose the final selection, “Waiver approved.” When doing this:
- Approval must be obtained from Dr. Sheila Vrana.
- Dr. Vrana’s approval must be saved as a PDF file and attached.
- The submission must include the reason why the waiver is approved or reference a published NIH guideline.

Administrative Expenses
Administrative expenses (such as office supplies and clerical supplies) may not be directly charged when federal funds are used, except in special circumstances.
The University offers additional details regarding such F&A exceptions.
Cost-Sharing
If the sponsor does not cover the PI salary, cost-sharing will be needed to move the study forward.
The University offers more information on cost-sharing.
All cost-sharing must be approved by the applicable department chair in writing. Investigators may not know up front whether cost-sharing is needed. If “No” is selected initially, it can be corrected at a later time when the need is identified.
If “Yes” is selected for cost-sharing, ensure that an approval document is attached in the Attachments tab.

Personnel
List all faculty members involved, including role.
Effort
Complete for principal investigator.
For industry-sponsored, if 1 percent, then enter .12 for PI. If 2 percent, then enter .24.
Refer to the conversion chart for IAF effort.
Credit
Award credit to all key personnel.
The “% Credit” column must total 100 percent.

Abstract
Enter the application abstract here.

Scientific Areas
For the College of Medicine, use the “Life Sciences” category. The 100 percent can be split between the Life Sciences subcategories.

Consult with the PI, coinvestigator(s), study coordinator and/or department research administrator to determine which program areas are applicable to the study.
Check all applicable areas.

The second question on the Export tab will be auto-populated based upon the name/address entered for the sponsor.
Each question must be answered accurately. If in doubt, check with the PI or other personnel (coordinator, sponsor contact if applicable, etc.) Don’t guess.

This is completed by investigators and coordinators as part of the workflow process.
On the Waiver tab, if either “Unrestricted grant” or “Gifts and charitable contributions” is selected, then the investigator and key personnel will be asked three additional questions when completing the Disclosures tab.

If “Yes” is chosen for “Does any portion of the project involve the use of human participants in research?” use the Import Study button to link to and import the IRB information. This is done after IRB approval. Do not import pending approvals.
Protocol on this tab actually means the IRB approval.
The grey box will not contain any information until the study is imported following IRB approval.
Workflow can be started without IRB approval. However, IRB approval is needed prior to award.
Please note that studies determined by the IRB to be human subjects exempt and studies being managed by an external IRB will not have an expiration date.

Vertebrate Animals
If choosing “yes” to whether vertebrate animals are used, a current IACUC approval is required.
A box will appear allowing the import of the IACUC approval. “Protocol” on this tab actually means the IACUC approval.

Biohazardous Materials
If choosing “yes” to whether biohazardous materials are used, a current biohazard safety approval is required.
The import study feature will not work for biosafety approval. The information must be added manually.
“Protocol” on this tab actually means the biosafety approval.
“Study #” is not the CATS IRB number, but instead is a unique number assigned by the Institutional Biosafety Committee (IBC).
The approval letter (e-mail) from the IBC will contain this unique number along with an expiration date.

This tab initially shows two questions, but selecting “Yes” to the first question will result in additional selections.

Please reach out to the Radiation Safety Committee (RSC) for all questions involving radioactive materials, radiation producing instruments, or lasers by contacting Heather Barber via hhb104@psu.edu or Brian Lorah via bjl14@psu.edu.
If selecting “Yes” to the question, “Does the research involve the use of radioactive materials, radiation-producing instruments, or lasers?”, you will be prompted with three additional questions.

If selecting “Yes” that radioactive materials are used, then a current radiation approval is required.
The “Import Study” feature will not work for radiation approval. This information must be added manually.


“Study #” is not the CATS IRB number, but instead is a unique number assigned by Radiation Safety Committee (RSC).
“Study Owner” is the investigator who received approval from the RSC to manage the radioactive materials. This may be an investigator other than the PI.
“Title” will be either the general approval name for the investigator approved to manage the radioactive materials or a project specific title.
There will be an expiration date given and this needs to be placed into the IAF.
Warning Message
A warning message that appears at the top of the “Attachments” Tab about not uploading award documents should be ignored. Those instructions are applicable to projects at University Park only, not to the College of Medicine.

What to Attach
- For “Full Proposal Upload,” do not upload anything. ORA will upload the submitted application.
- Under “Add Other Proposal Document,” include any cost-sharing approval document, if applicable, and choose the appropriate type and enter a description.
- Under “Add Other Proposal Document,” include any Confidential Disclosure Agreement (CDA), if applicable, and choose the appropriate type and enter a description.
Maximum document size is 100MB.
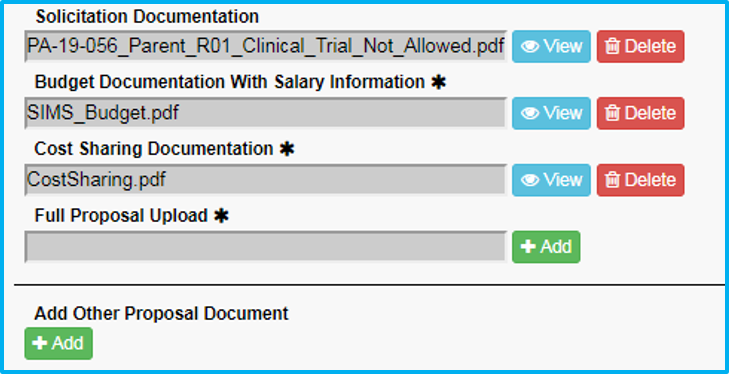
Verify all required fields/questions have been completed by clicking on the “Summary” tab.
Any required field that is not complete will include a “Not Answered” box with a yellow background.
If an investigator (including the primary investigator) is removed after the workflow has been started, it will be noted on this tab.
Once the IAF is a “pending proposal,” the investigator’s and key personnel’s responses to the Disclosure tab will be visible on the summary.

The workflow should be started before bringing the grant to ORA for review.
After approving an IAF, the user will be redirected to the IAF Approval Confirmation Page.
Once approved by the PI, the approvals are workflowed to any co-investigators simultaneously.

Submit a Proposal Screen
To submit an IAF for RPPR (NIH annual progress reports), on the “Submit a Proposal” screen, select “A Non-Competing Continuation.”

After choosing that, there will be a prompt to enter the existing OSP number. Enter the number and click the red magnifying glass to verify. When the magnifying glass turns green, use the “Create New IAF from Existing” button to continue.
The IAF will populate with the answers from the previous year.
Budgets
For an RPPR, the budget tab will not prepopulate.
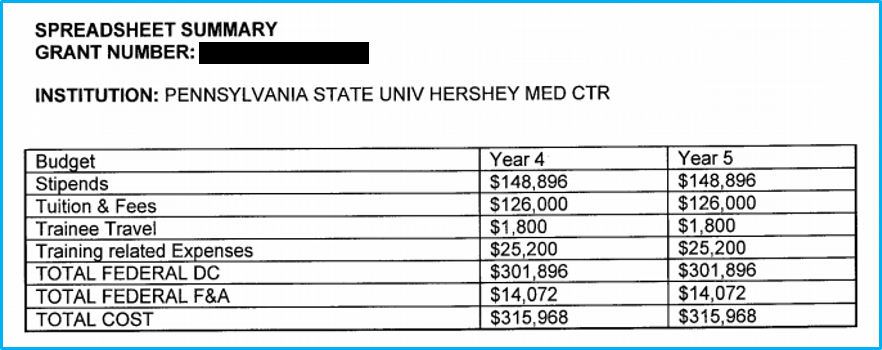
Open the Current Statement of Award.
Find the budget breakdown “Spreadsheet Summary.”
- Direct: Enter the “Total Federal DC” number from the spreadsheet.
- F&A: Enter the “Total Federal F&A” number from the spreadsheet.
- Total: Enter the “Total Cost” number from the spreadsheet.
In an example of the spreadsheet summary, with columns for Year 4 and Year 5 visible, if Year 4 represents the current year, the Year 5 info is what should be used for the RPPR.
For federal resubmissions or continuations, go to the Budget tab and enter the grant number.
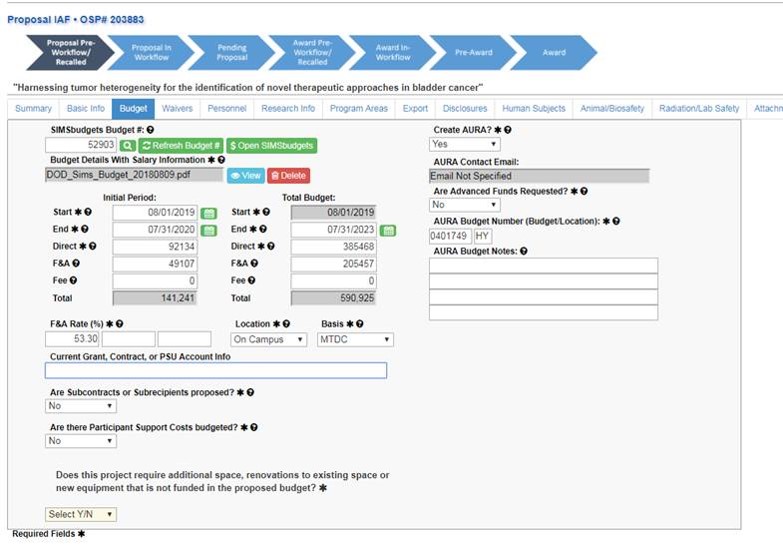
Submit a Proposal Screen
To submit an IAF for resubmission, on the “Submit a Proposal” screen, select “Resubmission of a Prior Proposal.”

After choosing that, there will be a prompt to enter the existing OSP number. Enter the number and click the red magnifying glass to verify. When the magnifying glass turns green, use the “Create New IAF from Existing” button to continue.

The IAF will populate with the answers from the original application.
Add the SIMS budget number and click the green magnifying glass.
Click “Refresh Budget #” to pull the budget information into the application.
Submit a Proposal Screen
To submit an IAF for a competitive renewal, on the “Submit a Proposal” screen, select “A Competitive Renewal.”

After choosing that, there will be a prompt to enter the existing OSP number. Enter the number and click the red magnifying glass to verify. When the magnifying glass turns green, use the “Create New IAF from Existing” button to continue.

The IAF will populate with the answers from the original application.
Add the SIMS budget number and click the green magnifying glass.
Click “Refresh Budget #” to pull the budget information into the application.

IAF training is available. Email e-grants@pennstatehealth.psu.edu or e-contracts@pennstatehealth.psu.edu as appropriate for details or to schedule.
Also, please ensure anyone who is listed on an IAF has their Penn State (@psu.edu) email forwarded to their Penn State Health (@pennstatehealth.psu.edu) email account so they receive the IAF alerts. See instructions for setting up email forwarding.
More IAF Details and Tips
If the gift, charitable grant or unrestricted grant requires an IAF (see Research Administration University Development Collaborative Procedures for Penn State College of Medicine and Penn State Health Gifts/Charitable Grants/Unrestricted Grants):
Unless otherwise indicated, complete all sections of the IAF as usual.
Basic Info
- Type of Submission: Select “New”
- Type of Project: Select “Research” or “Instruction” or “Outreach” – never select “Clinical Trial”
- Remarks: Include standard language from ORA – “Gift/Charitable Grant/Unrestricted Grant following process established under RAUD Collaborative Procedures – Hershey Addendum.”
Budget
- Initial Period: Enter a project period of one year
- Total Budget: Should be the same as Initial Period
- F&A Rate
- Gifts: 0 percent
- Charitable grants: 15 percent (in absence of published guidelines)
- Unrestricted grants: 15 percent
- Are Advanced Funds Requested? Select “No”
Waivers
- Type of F&A
- If 0% percent F&A, select “None”
- If 15 percent F&A, select “Partial”
- If the budget indicates less than full F&A, please select why? Select “Gifts and charitable contributions”
Personnel
- No effort required
Research Info
- Abstract: Add a few general statements about the gift or unrestricted grant or charitable grant
- Program Areas: No special instructions
- Export: No special instructions
- Under Disclosure, Additional questions for Gift or Unrestricted Grant or Charitable Grant (This section is completed by the Investigator(s) and not visible to others):
- Is the funder expecting anything beyond a stewardship report in exchange for financial assistance (e.g., detailed technical/financial report; IP or data rights; test results; prototypes; restriction on publication? Select “Yes” or “No”
- If yes, provide additional explanation
- Have you, your spouse, partner, and/or dependent children, currently or in the past year, had agreements with this funder (including consulting agreements, non-disclosure agreements, gifts, and/or sponsored research agreements)? Select “Yes” or “No”
- If yes, provide additional explanation
- Have you, your spouse, partner, and/or dependent children, currently or in the past year, had any other financial or non-financial relationships with this funder (e.g., sitting on a board of directors, ownership of equity)? Select “Yes” or “No”
- If yes, provide additional explanation
- Is the funder expecting anything beyond a stewardship report in exchange for financial assistance (e.g., detailed technical/financial report; IP or data rights; test results; prototypes; restriction on publication? Select “Yes” or “No”
Human Subjects
- If project involves Human Subjects, will require IRB approval and will require approval by compliance office (HSPO)
Animal/Biosafety
- If project involves animal subjects, will require IACUC approval and will require approval by compliance office (IACUC)
- If project involves biohazardous materials, will require Biosafety approval and will require approval by compliance office
Radiation/Lab Safety
- If project involves radiative materials, radiation-producing instruments or lasers, will require approval by compliance office
Attachments Tab
- Budget: Upload a copy of the check
- Full Proposal Upload: Upload a blank document that states “No proposal submitted”
- Other: Upload any email explanation/correspondence of the purpose of the gift or the cover letter that came with the check.
Next Steps
Once the IAF is complete with uploaded documents and in workflow, email e-grants@pennstatehealth.psu.edu and controller@pennstatehealth.psu.edu with the IAF/OSP number and a brief description to alert those teams that it is ready for processing.
Standard Process for Proposals
The following guidelines are for a standard submission process. Use Google Chrome or Mozilla Firefox browsers while working in the IAF system.
- Department research administrator starts the IAF. In consultation with the principal investigator, this administrator completes all information except for the Investigator tab. Capitalize the first letter of each word in the title with the exception of articles (in, an, the, etc.).
- The department research administrator puts the IAF into workflow.
- A copy of the first page of the IAF, copy of application, budget and RFA are brought to the Office of Research Affairs.
- The principal investigator is automatically sent an email notifying them that an IAF is awaiting their input and approval. The PI fills out the Investigator tab, fills out any additional inputs that the research administrator did not complete, then reviews and approves the entire IAF. At any time after an IAF has been created, the PI can fill in as much of the IAF as they would like. This would allow a PI to provide their inputs if they will not be available just prior to proposal submission time when they would normally fill it out. Any co-investigators and the PI’s department chair are automatically sent an email notifying them that an IAF is awaiting their input and approval.
- Co-investigators fill out the Investigator-only tab and review and approve the entire IAF. The co-investigators’ department heads are automatically sent an email notifying them that an IAF is awaiting their approval as each co-investigator approves.
- Department chairs and any additional approvers review and approve the IAF.
- The departmental research administrator will email a final PDF of the application to their grants officer. The Office of Research Affairs then reviews, approves and submits to the Office of Sponsored Programs at the University.
- The PI and all investigators with individual compliance issues will receive a compliance checklist.
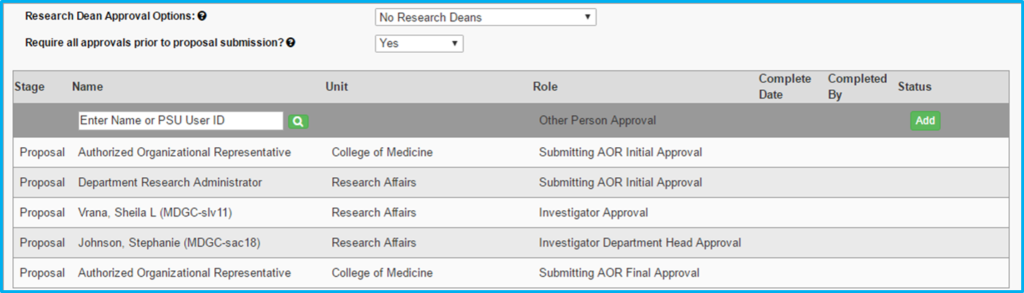
Proposal Approvals
The following people will always be required to approve the IAF, although not necessarily before proposal submission:
- Principal investigator
- Co-investigators
- PI’s and co-investigators’ department chairs
- Institute director (if submitted through an institute)
If the sponsor requires PI certification prior to proposal submission (e.g., NIH), the submitting departmental research administrator is responsible for ensuring compliance. If changes are made to the IAF after an approval, the requirement for obtaining a re-approval from the same individuals is at the discretion of the submitting administrator.
Additional approvals may also be required at the discretion of the submitting AOR. These approvals may include:
- Department chair of PI and co-investigators (for example, in cases involving cost-sharing, buyout, facility requirements, overhead waiver or significant equipment purchase)
Recalls and Revisions
IAFs can be recalled after being sent into workflow. Anyone awaiting approval will receive an email notifying them that the IAF cannot be approved. Once the ePIAF has been re-sent into workflow, the pending approvers will receive an email notifying them that the ePIAF can now be approved.
Do not recall an IAF without consulting with the Office of Research Affairs first.
The following changes to an IAF will require the IAF to be rerouted for approvals:
Proposal Types
There are some situations that may alter the standard approvals required as listed here:
General Approval Rules
- Department chairs may delegate their approval. Investigators may not.
- No one can approve in more than one role.
- There are special situations for investigators who hold another role, such as department chair.
Detailed Budget Information
Detailed budgets including salary information will be uploaded to the IAF.
Only the PI, the PI’s department head and the Office of Research Affairs will have access to the budget.
A separate personnel page that shows all named personnel and their percentage of effort and percentage of credit will be available for those without detailed budget access.
An advanced fund request cannot happen until the IAF has been submitted by ORA and is a pending proposal. Once that happens, to start an advanced fund request, submitters should do the following:
- Click “Create IAF” in the IAF system.
- Select “Start an Advanced Fund Request.”
- Place IAF number in the OSP lookup and hit the red magnifying glass.
- Once IAF is verified, click “Create New IAF from Existing.”
- Select “Yes” to Advanced Funds Requested.
- Add the cost center info into the Cost Center field.
- Save.
- Place IAF back into workflow.
- Check the boxes next to the two statements. Please note that by checking the statements, the department is acknowledging they are responsible for these funds if award does not materialize. The statements note that the submitter certifies that:
- “I am aware that Advanced Funds will be requested during award processing;” and
- “I understand and acknowledge that any and all financial and legal risks and/or liabilities which may result from starting work on the project prior to final award execution will be the responsibility of the requesting College, Department and/or Unit.”
- Click “I Approve.”
- Contact ORA once this has been done so the IAF can be submitted to ORA for the final step.
- The SIMBA account will not generate until ORA submits and compliance is approved.
IAF Support
For IAF questions, contact Marybeth Brown at 717-531-8495 or mbrown2@pennstatehealth.psu.edu or Brenda Thomas at 717-531-4196 or bthomas2@pennstatehealth.psu.edu.
