The Center for Medical Innovation offers a number of resources, programs and services that educate and provide assistance to faculty, staff and students in identifying and implementing the best strategies for moving their discoveries beyond the lab and into the commercial arena.
Services provided include:
- Generating a business strategy
- Providing access to funding
- Assess intellectual property for patentability
- Intellectual property protection: CDAs, NDAs, BMLAs, patents, copyrights, trade and service marks
- Entrepreneur mentoring
- Marketing intellectual property to potential investors or industry partners
- Facilitate networking
- Educational seminars, workshops
- New venture support
Jump to topic
Search
CMI Resources
Innovation Abstract
The innovation abstract form is the first step towards commercialization. It provides the Center for Medical Innovation general information about you and your idea, what problems you are addressing, what makes your innovation unique and what kind of help is desired.
Invention/Technology Disclosure
An invention disclosure is more than a form to fulfill a University Policy; it is the first official record of a technology. An invention disclosure allows an innovator to share limited information about the technology with the Center for Medical Innovation to allow its staff to examine commercial potential. Filling an invention disclosure can be the first step in the intellectual property protection process.
CDA (Confidential Disclosure Agreement)
A CDA is a legal agreement between two or more parties that provides terms under which information shall be shared. CDAs are important to prevent premature public disclosure of information, which can limit or even preclude patent rights.
Clinical Trial and Basic Science CDAs are processed through the Office of Research Affairs (ORA).
Intellectual Property (IP) CDAs to license existing Penn State technology are processed through the Center for Medical Innovation (CMI).
Submit Clinical Trial or Basic Science CDA Request to ORA
Submit Intellectual Property/Licensing CDA Request to CMI
MTA (Material Transfer Agreement)
An MTA provides the terms for the use of tangible research materials such as the most commonly negotiated elements; rights and ownership of intellectual property, publication rights, liability and governance. The MTA describes the terms under which Penn State and Penn State Health Milton S. Hershey Medical Center researchers can share materials, typically for research or evaluation purposes.
Ordering from BEI or ATCC
Penn State College of Medicine has a Master Agreement in place with both BEI and ATCC. Note that the PI is required to sign an Acknowledgement of Material Transfer Agreement form when placing an order with either organization. Contact the CMI for the acknowledgement form or for more information about the process.
Intellectual Property Policies
- IP01 – Ownership and Management of Intellectual Property
- IP02 – Co-Authorship of Scholarly Reports, Papers and Publications
- IP03 – Courseware
- IP04 – Royalty Payments for Course Materials
- IP05 – Policy Governing Copyright Clearance
- IP06 – Technology Transfer and Entrepreneurial Activity (Faculty Research)
Intellectual Property Guidelines
- IPG01 – Faculty Guidance on Student Intellectual Property Rights
- IPG02 – Special Student Intellectual Property Agreement Forms
- IPG03 – What to Expect When Licensing a Penn State Technology into a Start-Up Company
- IPG04 – Acceptance of Donated Intellectual Property by The Pennsylvania State University
Review and Management of Conflict of Interest
For medical innovations, the journey from ideation to clinical implementation is equally complex and demanding. There are many hurdles to overcome and milestones to consider, each requiring a distinct set of resources. CMI is here to guide you through each chapter that will make up your unique development story.
FDA Considerations to Fast-Track Your Translational Research
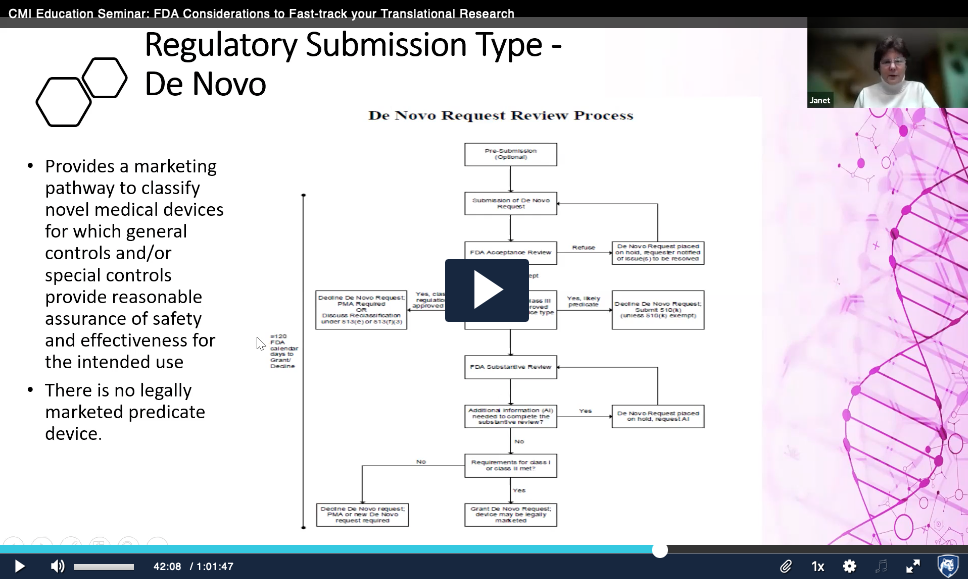 Recorded Jan. 11, 2023 – In this seminar two leaders from industry discuss the basics of FDA regulations pertinent to therapeutics and medical devices. They share key elements that early-stage innovators should consider throughout the developmental pipeline, including early bench research and preclinical studies.
Recorded Jan. 11, 2023 – In this seminar two leaders from industry discuss the basics of FDA regulations pertinent to therapeutics and medical devices. They share key elements that early-stage innovators should consider throughout the developmental pipeline, including early bench research and preclinical studies.
Protecting Your Intellectual Property (IP)
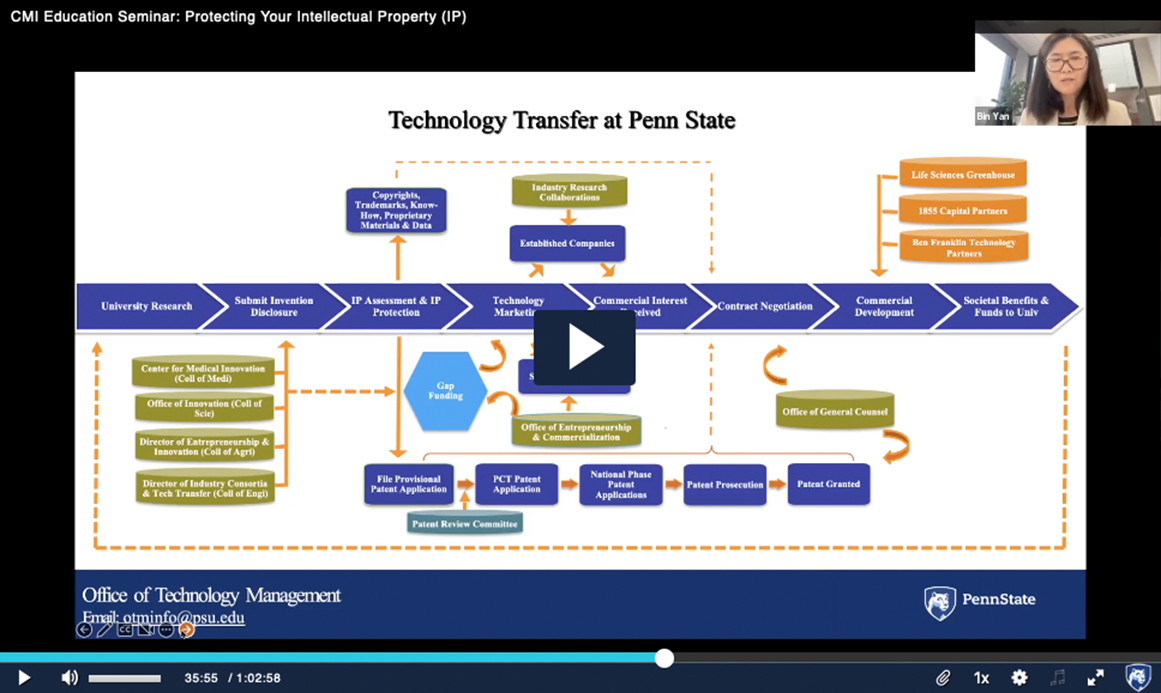 Recorded Nov. 11, 2022 – Our experts provide an overview of what constitutes intellectual property, recent changes to US Patent and Trademark Office (USPTO) guidelines and how IP can impact the future marketability with investors and industry partners. They also give an introduction to the IP protection and technology licensing processes at Penn State as well as resources available to help bring your technology toward commercialization.
Recorded Nov. 11, 2022 – Our experts provide an overview of what constitutes intellectual property, recent changes to US Patent and Trademark Office (USPTO) guidelines and how IP can impact the future marketability with investors and industry partners. They also give an introduction to the IP protection and technology licensing processes at Penn State as well as resources available to help bring your technology toward commercialization.
Funding Life Science Innovations and Startups
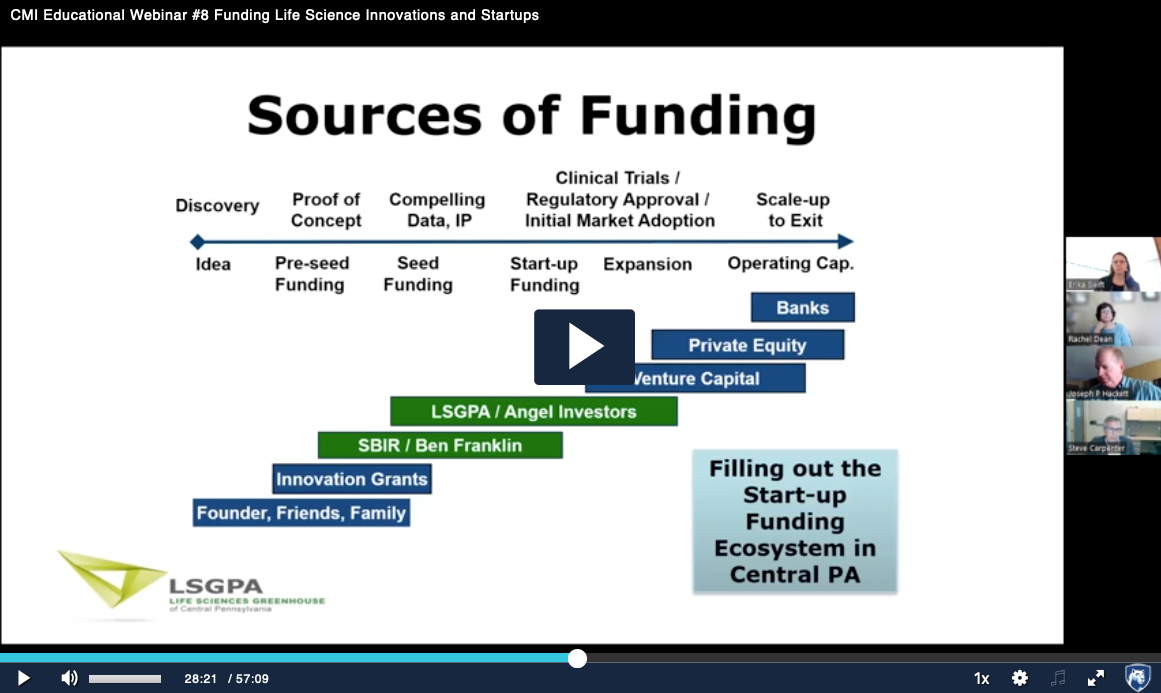 Recorded Oct. 14, 2022 – Innovative technology development and advancement requires significant investments in funding and resources. In this video our panel of experts provide an overview of various external funding opportunities available and provide insight in how to apply and position your translational research and technology for long-term success.
Recorded Oct. 14, 2022 – Innovative technology development and advancement requires significant investments in funding and resources. In this video our panel of experts provide an overview of various external funding opportunities available and provide insight in how to apply and position your translational research and technology for long-term success.
Penn State Resources for Technology Development
 Recorded Sept. 29, 2022 – Do you have an idea for a medical innovation such as novel implant, surgical instrumentation, wearable, device, therapeutic or diagnostic? Discover Penn State resources that can help identify collaborators with specific expertise, as well as support the design, development, prototyping and testing of potential solutions.
Recorded Sept. 29, 2022 – Do you have an idea for a medical innovation such as novel implant, surgical instrumentation, wearable, device, therapeutic or diagnostic? Discover Penn State resources that can help identify collaborators with specific expertise, as well as support the design, development, prototyping and testing of potential solutions.
Academics to Industry: Translational Development Pathways for Medical Innovations
 Recorded Oct. 21, 2021 – Hear an overview of the multi-faceted translation process of moving basic research and clinical innovations from an academic institution to industry. Discover the best ways to utilize key internal resources and optimize strategic partnerships outside of the university to set your project up for success.
Recorded Oct. 21, 2021 – Hear an overview of the multi-faceted translation process of moving basic research and clinical innovations from an academic institution to industry. Discover the best ways to utilize key internal resources and optimize strategic partnerships outside of the university to set your project up for success.
Protecting Intellectual Property for Research Innovations
 Recorded Nov. 18, 2021 – We provide an overview of what constitutes IP, what types of IP may benefit from patent protection and how strong IP can impact the future marketability with investors and industry partners. You also receive an introduction to the IP protection process at Penn State and learn about resources available to bring your technology toward commercialization.
Recorded Nov. 18, 2021 – We provide an overview of what constitutes IP, what types of IP may benefit from patent protection and how strong IP can impact the future marketability with investors and industry partners. You also receive an introduction to the IP protection process at Penn State and learn about resources available to bring your technology toward commercialization.
Early Regulatory Processes in Translational Research
 Recorded Dec. 16, 2021 – Understanding the regulatory requirements and expectations of drug and device development allows for the design of more efficient, yet comprehensive, clinical protocols. In this seminar, we discuss Penn State’s human subject research processes and the importance of long-term strategic planning. Our experts share how they work with researchers to support translational studies by advancing clinical trial protocols that meet regulatory expectations.
Recorded Dec. 16, 2021 – Understanding the regulatory requirements and expectations of drug and device development allows for the design of more efficient, yet comprehensive, clinical protocols. In this seminar, we discuss Penn State’s human subject research processes and the importance of long-term strategic planning. Our experts share how they work with researchers to support translational studies by advancing clinical trial protocols that meet regulatory expectations.
Human-Centered Design Thinking for Medical Innovations
 Recorded Feb. 24, 2022 – An important part of the journey from idea to clinical use is understanding who will use, buy and benefit from your innovation. In this video, our experts discuss using Customer Discovery and hypothesis testing to identify key customers, refine the commercial market for your technology and uncover the information that would allow you to design a suitable solution, thus improving the odds of success.
Recorded Feb. 24, 2022 – An important part of the journey from idea to clinical use is understanding who will use, buy and benefit from your innovation. In this video, our experts discuss using Customer Discovery and hypothesis testing to identify key customers, refine the commercial market for your technology and uncover the information that would allow you to design a suitable solution, thus improving the odds of success.
Corporate and Foundation Funding Mechanisms
 Recorded March 17, 2022 – Development and advancement of innovative technologies requires significant funding and resource investments. In this seminar, we explore how to utilize corporate and foundation funding to advance medical innovation. By developing and maintaining partnerships between Penn State and many external funders, our experts outline how to build strategic long-term relationships and identify areas of mutual opportunity.
Recorded March 17, 2022 – Development and advancement of innovative technologies requires significant funding and resource investments. In this seminar, we explore how to utilize corporate and foundation funding to advance medical innovation. By developing and maintaining partnerships between Penn State and many external funders, our experts outline how to build strategic long-term relationships and identify areas of mutual opportunity.
Talking About My Science to the Non-Scientist
 Recorded April 21, 2022 – Current communication channels for presenting scientific research have expanded and understanding how to effectively address each outlet can be challenging. Traditional academic research conferences require one approach, while opportunities to procure funding require a different set of delivery expectations. In this video, our experts provide examples and best practices for effectively engaging these various audiences.
Recorded April 21, 2022 – Current communication channels for presenting scientific research have expanded and understanding how to effectively address each outlet can be challenging. Traditional academic research conferences require one approach, while opportunities to procure funding require a different set of delivery expectations. In this video, our experts provide examples and best practices for effectively engaging these various audiences.
BioStrategy Partners Practical Knowledge Series Webinars
 The CMI collaborates with BioStrategy Partners to educate the local community about the commercialization and technology transfer process through their Practical Knowledge Series (PKS) webinars. They provide a forum for bringing content-area experts together with members of the academic, technology transfer and entrepreneurial communities to share information, experience and insights on topics related to commercialization of life sciences technologies.
The CMI collaborates with BioStrategy Partners to educate the local community about the commercialization and technology transfer process through their Practical Knowledge Series (PKS) webinars. They provide a forum for bringing content-area experts together with members of the academic, technology transfer and entrepreneurial communities to share information, experience and insights on topics related to commercialization of life sciences technologies.
Topics include:
- Customer Discovery: Building Something Someone Wants
- Principles of Company Formation
- Building for a Successful Exit
- Clinical Trial Design: How it Impacts Outcomes
- Accelerating Growth through Strategic Partnerships
- Reimbursement and Health Economics
- Funding sources: Angels, SBIRs, Tax credits
- Fundamentals of IP Management
- And more!
View the complete PKS video library on the BioStrategy Partners YouTube Channel.
I-Corps@NCATS Program
Offered in partnership with Penn State Clinical and Translational Science Institute, this course is based on the Innovation Corps (I-Corps) curriculum and offers a methodology to help discover the commercial potential technology. During the course, participants will learn how to explore product market fit, talk to customers and gain insight from experienced leaders.
Learn more about I-Corps@NCATS
Innovation Fellows Program
The Innovation Fellows Program is a 12-month, non-clinical program that admits multiple fellows per year on a competitive basis. It is designed to provide qualified young scientific leaders who have an interest in translation, commercialization and business disciplines to align with an innovative faculty member and further develop a new healthcare invention to address an unmet medical need.
Learn more about Innovation Fellows
CMI Proof of Concept Program
The CMI Proof of Concept Program aims to identify translational research and early-stage technologies that are addressing an unmet medical need. Through seed funding and project management CMI helps rapidly develop the innovations through a milestone-driven process that will generate data needed to apply for follow on funding opportunities.
Learn more about Proof of Concept Program
CMI Innovation Accelerator Program
The CMI Innovation Accelerator Program supports College of Medicine Primary Investigators accomplish milestone-driven success towards commercialization of an innovative technology. Projects will receive funding and business development support by partnering with the CMI team in advancing the technology through the duration of the award.
Learn more about Innovation Accelerator Program
Innovation Café
Innovation Café is a regional event focused on expanding and sustaining the central Pennsylvania entrepreneurial ecosystem. This reoccurring networking forum features high profile guest speakers discussing industry innovations, business trends, regulations and the impact on the life sciences and healthcare community. The Innovation Café is a great place to learn about the latest innovations while networking with entrepreneurs, investors, economic development organizations, government agencies and community members.
Ben Franklin Techcelerator Program
The Techcelerator program offers startup accelerators and entrepreneurial training for regional entrepreneurs, students, faculty members and small business owners. The innovative program contributes to an entrepreneurial climate, increases technology transfer opportunities and helps develop a robust startup culture at Penn State and the surrounding area.
Learn more about Techcelerator
Innovation Partnership
This initiative provides technical and business review for all SBIR/STTR grant applications at no charge to you.
Biostrategy Partners
Biostrategy Partners is a nonprofit consortium of academic medical centers and research institutes committed to the development and transfer of academic research into the marketplace by developing and offering programs and services to foster industry-academic collaboration, aid technology development and commercialization and contribute to the ongoing education of faculty and graduate students about the commercialization and technology transfer processes.
Learn more about Biostrategy Partners
Lending Library
The Center for Medical Innovation offers loan of books covering topics such as innovation, entrepreneurship, leadership and building a start-up company. Contact us for a list of available titles.
Available Technologies
A wide portfolio of technologies is available through Penn State University. Explore a list of patented technologies here.
Core Facilities
Penn State College of Medicine offers a variety of shared-service core research facilities. Explore information on core facilities here.
Invent Penn State
Invent Penn State blends entrepreneurship-focused academic programs, business startup training and incubation, funding for commercialization, and university-community collaborations to facilitate the challenging process of turning research discoveries into valuable products and services that can benefit Pennsylvanians and humankind.
Penn State Office of Technology Management
The Office of Technology Management is responsible for managing, protecting and licensing the intellectual property of faculty, graduate students and staff at all Penn State locations.
Penn State College of Medicine Research
Penn State College of Medicine strives to be a national leader in pursuing basic, clinical, translational and health services research, and developing programs to advance medical and scientific knowledge. Explore more College research initiatives here.
Inside Entrepreneurship Law
Inside Entrepreneurship Law is an educational blog from Penn State Dickinson Law designed to aid entrepreneurs and their counsel by providing information on relevant topics.
U.S. Patent and Trademark Office
As a mechanism that protects new ideas and investments in innovation and creativity, the USPTO is the federal agency for granting U.S. patents and registering trademarks.
Google Patents
Search and read the full text of patents from around the world on Google Patents, and find prior art in Google’s index of non-patent literature.
U.S. Copyright Office
Search the Copyright Public Records Catalog online dating back to 1978, as well as a searchable database of court opinions, including by category and type of use, via the U.S. Copyright Office.
WIPO
The World Intellectual Property Organization (WIPO) is the global forum for intellectual property services, policy, information and cooperation.
SBA
The U.S. Small Business Administration has delivered millions of loans, loan guarantees, contracts, counseling sessions and other forms of assistance to small businesses.
