The Surgical Innovation Group (SIG) has been serving Penn State Health, College of Medicine and the university for more than 10 years with the aim of accelerating the overall pace of surgical innovation by drawing on the expertise of clinicians, researchers, staff and industry professionals. The focus of SIG is medical devices for surgical, orthopaedics, routine patient care, diagnostics and treatment applications, including instruments, apparatuses, implants, wearables and machines.
SIG specializes in leveraging organizational assets, including cutting edge technology, research discoveries and excellence in clinical care utilizing Penn State crowd-sourced problem-solving and multidisciplinary networking. See the complete list of complimentary services below.
Whether you have an idea for a new product or have a problem to solve, contact SIG today.
Contact SIG about your project, idea or clinical challenge
Jump to topic
Search
Learn More about the Surgical Innovation Group
Just like research, innovation is a process and a discipline. It all starts with an idea or challenge, but that is only the first step. The goal of the Surgical Innovation Group (SIG) is to create a forum to understand the clinical problem, expand ideas, and ideate on solutions with market considerations in mind. Robust project management efficiently moves potential solutions forward along the commercialization pathway facilitating getting products to market faster where they can improve health and benefit society.
SIG uses a collaborative approach to vet ideas and develop promising medical innovation using an iterative process, preparing technologies for exit through strategic partnership, license or startup. The group has extensive experience moving medical technologies towards market utilizing our broad network to de-risk solutions and determine potential patient impact. SIG is passionate about helping you solve a problem and turning your ideas into tangible, real-world products.
The Surgical Innovation Group is a collaboration between the Department of Surgery and the Center for Medical Innovation, drawing on members’ vast networks of multidisciplinary and industry contacts.
The Surgical Innovation Group aims to move your medical innovation from ideation to clinical implementation. Key services, offered to propel you along the commercialization pathway, include:
- Assess and refine technologies leveraging expertise in design and engineering
- Prototyping and 3D printing services
- Expanding Intellectual Property (IP)
- Connect with an extensive network of multidisciplinary collaborators
- Entrepreneurial advisors and mentors
- Project management guidance
- Funding opportunity support
- Link your technology to industry partners
- De-risk technologies for attracting investors
- Marketing, graphics, illustrations and other various visualizations
- Regulatory guidance
- Provide access to a comprehensive team of clinical and engineering faculty
- Customer discovery, market analysis and business strategy
AdvantageRib

AdvantageRib
- Read about the development of AdvantageRib
- Read about the acquisition by Zimmer Biomet
- Watch a video showing the innovative technology in action
Cranial Devices
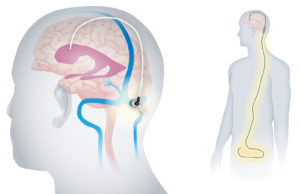
Cranial device
Ramiah’s Story
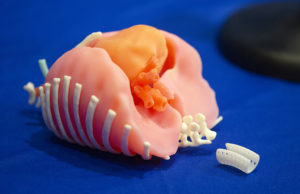
Model to size doctors used to create 3D part right for Ramiah Martin.
- Read more about Ramiah’s story in this article
- View more photos with Ramiah’s story at Penn State News
- View a photo gallery about Ramiah from Pennlive
MASC Initiative

Mask designs
As Penn State Health was strategically preparing to face the impact of COVID-19, a multidisciplinary team assembled to form MASC, the Manufacturing and Sterilization for COVID-19, an initiative focused on designing and delivering rapidly scalable solutions to solve clinical needs for Penn State and health systems across Pennsylvania. Members of the Surgical Innovation Group were instrumental in progressing a number of the projects, including 3D-printed protective masks, disposable stethoscopes and test swabs, as well as partnering with local GMP manufacturers to help design, fabricate and deliver hundreds of thousands of pieces of personal protective equipment (PPE) such as face shields, masks and gowns.
 The Surgical Innovation Group is led by director Anthony Tsai, MD. Dr. Tsai is a pediatric surgeon, medical innovation facilitator, global health promoter and gadget enthusiast. With a special interest in digital health, artificial intelligence, augmented and virtual reality, health science systems, and innovation process, he seeks to promote health globally through the intersection of medicine, technology, innovation and faith.
The Surgical Innovation Group is led by director Anthony Tsai, MD. Dr. Tsai is a pediatric surgeon, medical innovation facilitator, global health promoter and gadget enthusiast. With a special interest in digital health, artificial intelligence, augmented and virtual reality, health science systems, and innovation process, he seeks to promote health globally through the intersection of medicine, technology, innovation and faith.
Dr. Tsai is currently an associate professor of surgery at Penn State Health Milton S. Hershey Medical Center, division of pediatric surgery. His main clinical interest is minimally invasive surgery, chest wall deformities and congenital malformations. He has led a successful implantation of a 3D printed splint in a patient with congenital airway defect that led to the second known North American survivor of the pathology, tracheal agenesis. He has published in the area of immunology, trauma and minimally invasive surgery. His research interest is in chest wall deformities, artificial intelligence, innovation process, surgical technologies and global health. He serves on the American Pediatric Surgical Association Industry and Institutional Advisory Committee and New Technology Committee.
Contact the director at atsai1@pennstatehealth.psu.edu.
Phone: 717-531-8521
Email: SIG@pennstatehealth.psu.edu
