The background image is Shown is a bottle of hemp oil with a dropper above it, with a blurred background of other medical marijuana products
Welcome to the Pennsylvania-approved Medical Marijuana Academic Clinical Research Center at Penn State
The Penn State Medical Marijuana Academic Clinical Research Center (ACRC) will become an internationally recognized expert in the benefits and dangers of medicinal cannabinoids and medical cannabis.
The goal of the center is to support the development of medical marijuana pre-clinical and clinical research and provide scientific evidence on the utility of medical cannabis. Currently, there are more than 30 researchers engaged in cannabis research within the ACRC; these researchers are divided into two divisions, a basic science division and a clinical science division.
Penn State College of Medicine was recognized as a potential ACRC site with the passage of ACT 16 in 2016, which legalized medical marijuana in Pennsylvania.
A key provision of this act was to “promote high-quality research” on medical marijuana.
In May of 2018, Penn State College of Medicine was one of eight universities approved by Gov. Tom Wolf as a Certified Academic Clinical Research Center. In June 2019, the Penn State College of Medicine ACRC, in a relationship with PA Options for Wellness, was one of the first three centers approved by the Commonwealth of Pennsylvania.
Director: Kent Vrana, PhD, Elliot S. Vesell Professor, Department of Pharmacology
Scientific Director: Wesley M. Raup-Konsavage, PhD, Assistant Professor, Department of Pharmacology
Basic Science Division
About
The basic science division utilizes pre-clinical models, such as in vitro studies and rodent models, to explore the potential therapeutic uses of cannabinoids for a variety of disease.
Current projects are exploring the efficacy of cannabinoids or cannabis to treat cancer, acute and chronic pain, anxiety, high blood pressure and post-traumatic stress disorder (PTSD).
Additional research within this group focuses on the chemistry of cannabinoids including pharmacokinetics, receptor binding and the development of novel cannabinoid-based compounds. These preclinical data will help to steer clinical research projects.
Basic Science Team
The basic science division utilizes pre-clinical models, such as in vitro studies and rodent models, to explore the potential therapeutic uses of cannabinoids for a variety of disease.
Current projects are exploring the efficacy of cannabinoids or cannabis to treat cancer, acute and chronic pain, anxiety, high blood pressure and post-traumatic stress disorder (PTSD).
Additional research within this group focuses on the chemistry of cannabinoids including pharmacokinetics, receptor binding and the development of novel cannabinoid-based compounds. These preclinical data will help to steer clinical research projects.
Clinical Science Division
Introduction
The clinical science division focuses on examining the benefits and potential harm of medical cannabis use for the current state-approved medical conditions.
Currently, there are two teams within this division. The first team is focused on health outcomes in patients currently taking medical marijuana for the treatment of state-approved conditions, with special attention on anxiety, post-traumatic stress disorder and pain. The second team focuses on clinical trials and they will be conducting double blind, placebo-controlled studies on the efficacy of cannabis to treat medical conditions and reduce reliance on opioids for pain.
Available Clinical Trials
Cannabidiol and Management of Endometriosis Pain
The study team will be looking at the effects of cannabidiol (CBD) in patients with endometriosis. It is believed that CBD will improve both pain and quality of life. The study will last a total of 12 weeks and involve several onsite visits in addition to daily pain assessments.
Contact: Kristin Riley, MD.
Clinical Science Team
The clinical science division focuses on examining the benefits and potential harm of medical cannabis use for the current state-approved medical conditions.
Currently, there are two teams within this division. The first team is focused on health outcomes in patients currently taking medical marijuana for the treatment of state-approved conditions, with special attention on anxiety, post-traumatic stress disorder and pain. The second team focuses on clinical trials and they will be conducting double blind, placebo-controlled studies on the efficacy of cannabis to treat medical conditions and reduce reliance on opioids for pain.
Cannabidiol and Management of Endometriosis Pain
The study team will be looking at the effects of cannabidiol (CBD) in patients with endometriosis. It is believed that CBD will improve both pain and quality of life. The study will last a total of 12 weeks and involve several onsite visits in addition to daily pain assessments.
Contact: Kristin Riley, MD.
Facilities
Penn State Medical Marijuana ACRC uses the following facilities to advance research in the field.
Super Critical CO2 Extractor
 Produces extracts from cannabis (or other dried plant material) using high pressure liquid CO2. This has the benefit of producing an extract that does not contain a solvent, because after the plant material is extracted, the CO2 quickly converts to a gas, leaving behind pure extract. This process is the most widely utilized in the hemp and medical marijuana industry.
Produces extracts from cannabis (or other dried plant material) using high pressure liquid CO2. This has the benefit of producing an extract that does not contain a solvent, because after the plant material is extracted, the CO2 quickly converts to a gas, leaving behind pure extract. This process is the most widely utilized in the hemp and medical marijuana industry.
Cannabinoid Drug Libraries
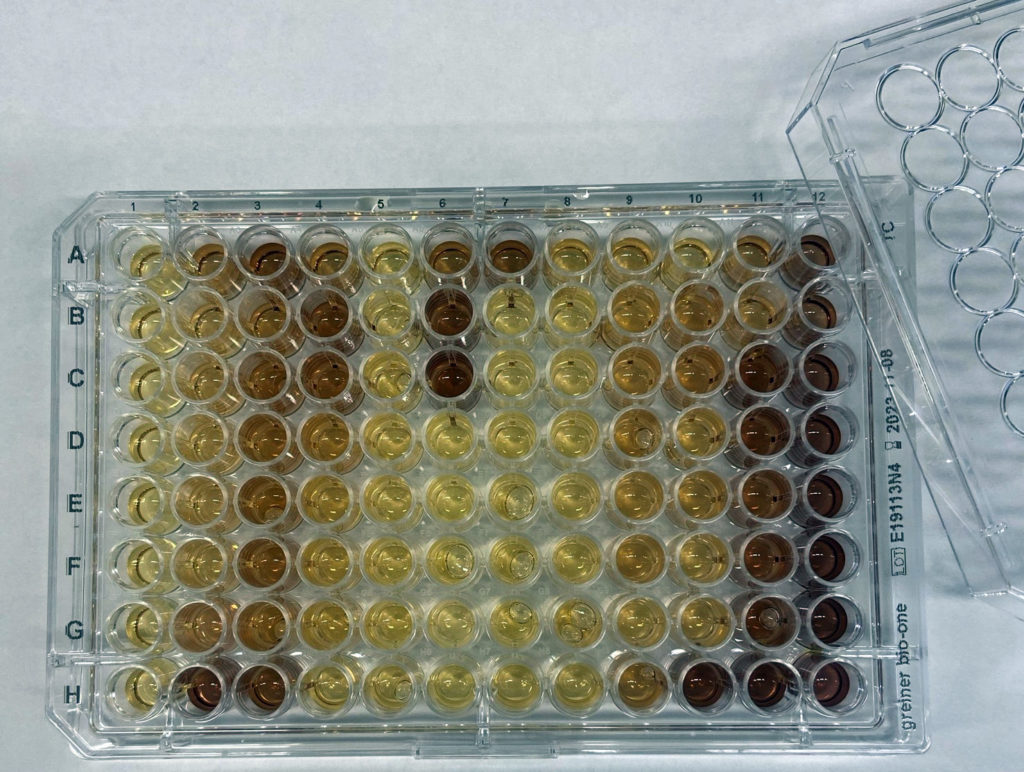 A library of approximately 500 synthetic cannabinoids and another library with approximately 60 endogenous cannabinoids can be used for in vitro screening of diseases and disease pathways. There is also a growing list of in house developed cannabinoid-based compounds created by the Organic Synthesis Core Facility.
A library of approximately 500 synthetic cannabinoids and another library with approximately 60 endogenous cannabinoids can be used for in vitro screening of diseases and disease pathways. There is also a growing list of in house developed cannabinoid-based compounds created by the Organic Synthesis Core Facility.
Other Reagents
 A number of other reagents are available for researchers looking to start work in the cannabis field. These include antibodies and RT-PCR probes to the major cannabinoid receptors, receptor agonists and antagonists and a small supply center for research team members.
A number of other reagents are available for researchers looking to start work in the cannabis field. These include antibodies and RT-PCR probes to the major cannabinoid receptors, receptor agonists and antagonists and a small supply center for research team members.


Scientific Director
Wesley M. Raup-Konsavage, PhD
Assistant Professor
Department of Pharmacology

Research Technician
Diana Sepulveda
Membership
The strength of the Center for Cannabis and Natural Product Pharmaceutics (CCNPP) is directly linked to bringing talented investigators from across the Penn State system. We encourage investigators interested in natural product pharmaceutics research collaborations to apply for the Penn State Center for Cannabis and Natural Product Pharmaceutics membership. Applicants must hold an appointment at Penn State, Penn State Health, a Penn State Medical Marijuana Academic Clinical Research Center (ACRC) partner or an affiliate institution.
Acceptance and/or approval of a membership application is determined by the leadership team.
Benefits of Membership
- Can request supercritical CO2 extraction of dried plant material
- Access to cannabinoids, natural product library (TimTec), and cannabis extracts
- Assistance with Grant Applications and Letters of Support
- Support for IRB, IND and IACUC protocol submissions
- Assistance developing tools for Mass Spectrometry, Microsome, and PK analysis
- Support for modification of lead compounds and formulation
Expectations and Responsibilities of Membership
- Acknowledgement of the CCNPP in publications
- Collaboration with members of the CCNPP
- Participation in CCNPP meetings
- Attendance at the annual CCNPP retreat
News

Publications
Events

Donate
Support natural product research at Penn State College of Medicine with a donation today
Millions of people currently take herbal supplements, including CBD oil or medical cannabis, that make claims to potential medical benefits. These statements and claims are rarely supported by strong scientific evidence, if any. Importantly, these supplements are not subject to government oversight, potential negative side-effects are not disclosed, and people believe they are safe (and therefore don’t mention to their primary care provider). The goals of the center are to identify novel natural product pharmaceutics, validate reported claims, and assess negative side-effects of natural products that consumers are using. Your generous gift helps to fund this work as well as to our work to educate clinicians on the medicinal value of plants and natural products.
Give hope today – Ways to give
Click here to donate online (credit card).
Make a gift by check payable to:
Penn State University – ACRC
Penn State Health Milton S. Hershey Medical Center
Penn State College of Medicine
Development & Alumni Relations, MC HS20
1249 Cocoa Ave, Suite 115
P.O. Box 852
Hershey, PA 17033-0852Have a question about giving?
Call 717-531-8497 or email giving@pennstatehealth.psu.edu.
Resources
New Tool for Evaluating Potential Drug-Drug Interactions Based on Cannabinoids
 CANNabinoid Drug Interaction Review (CANN-DIR®), developed at Penn State College of Medicine, is intended to evaluate for potential drug-drug interactions based on the cannabinoids (THC and CBD) affecting the metabolism of other concomitantly prescribed medications. This tool has been developed for use by healthcare providers, patients, caregivers and interested community members.
CANNabinoid Drug Interaction Review (CANN-DIR®), developed at Penn State College of Medicine, is intended to evaluate for potential drug-drug interactions based on the cannabinoids (THC and CBD) affecting the metabolism of other concomitantly prescribed medications. This tool has been developed for use by healthcare providers, patients, caregivers and interested community members.Additional resources
Newsletter
CCNPP newsletter
- View past newsletters in Sharepoint (PSU login required)
Sign up for the newsletter
Sign up for the Center for Cannabis and Natural Product Pharmaceuticals mailing list
The strength of the Center for Cannabis and Natural Product Pharmaceutics (CCNPP) is directly linked to bringing talented investigators from across the Penn State system. We encourage investigators interested in natural product pharmaceutics research collaborations to apply for the Penn State Center for Cannabis and Natural Product Pharmaceutics membership. Applicants must hold an appointment at Penn State, Penn State Health, a Penn State Medical Marijuana Academic Clinical Research Center (ACRC) partner or an affiliate institution.
Acceptance and/or approval of a membership application is determined by the leadership team.
Benefits of Membership
- Can request supercritical CO2 extraction of dried plant material
- Access to cannabinoids, natural product library (TimTec), and cannabis extracts
- Assistance with Grant Applications and Letters of Support
- Support for IRB, IND and IACUC protocol submissions
- Assistance developing tools for Mass Spectrometry, Microsome, and PK analysis
- Support for modification of lead compounds and formulation
Expectations and Responsibilities of Membership
- Acknowledgement of the CCNPP in publications
- Collaboration with members of the CCNPP
- Participation in CCNPP meetings
- Attendance at the annual CCNPP retreat


Support natural product research at Penn State College of Medicine with a donation today
Millions of people currently take herbal supplements, including CBD oil or medical cannabis, that make claims to potential medical benefits. These statements and claims are rarely supported by strong scientific evidence, if any. Importantly, these supplements are not subject to government oversight, potential negative side-effects are not disclosed, and people believe they are safe (and therefore don’t mention to their primary care provider). The goals of the center are to identify novel natural product pharmaceutics, validate reported claims, and assess negative side-effects of natural products that consumers are using. Your generous gift helps to fund this work as well as to our work to educate clinicians on the medicinal value of plants and natural products.
Give hope today – Ways to give
Click here to donate online (credit card).
Make a gift by check payable to:
Penn State University – ACRC
Penn State Health Milton S. Hershey Medical Center
Penn State College of Medicine
Development & Alumni Relations, MC HS20
1249 Cocoa Ave, Suite 115
P.O. Box 852
Hershey, PA 17033-0852
Have a question about giving?
Call 717-531-8497 or email giving@pennstatehealth.psu.edu.
New Tool for Evaluating Potential Drug-Drug Interactions Based on Cannabinoids
 CANNabinoid Drug Interaction Review (CANN-DIR®), developed at Penn State College of Medicine, is intended to evaluate for potential drug-drug interactions based on the cannabinoids (THC and CBD) affecting the metabolism of other concomitantly prescribed medications. This tool has been developed for use by healthcare providers, patients, caregivers and interested community members.
CANNabinoid Drug Interaction Review (CANN-DIR®), developed at Penn State College of Medicine, is intended to evaluate for potential drug-drug interactions based on the cannabinoids (THC and CBD) affecting the metabolism of other concomitantly prescribed medications. This tool has been developed for use by healthcare providers, patients, caregivers and interested community members.
Additional resources
CCNPP newsletter
- View past newsletters in Sharepoint (PSU login required)
Sign up for the newsletter
Sign up for the Center for Cannabis and Natural Product Pharmaceuticals mailing list
