About the lab
The interventional endoscopy team looks forward to further research and innovation, bringing premier leading-edge care to the patients of central Pennsylvania and developing tomorrow’s procedures today through research and publication.
Jump to topic
Search
Meet the Team

Matthew T. Moyer, MD, MS, FASGE
Professor of Medicine
Division of Gastroenterology and Hepatology
Penn State Cancer Institute
Dr. Moyer concentrates both clinically and in research on interventional endoscopy, gastrointestinal cancer, and the research and development of innovative endoscopic techniques and devices in these areas.
Since graduating his fellowship in 2009 and joining the nationally recognized interventional endoscopy group, Dr. Moyer has been heavily involved in developing the quality and volume of interventional endoscopy and endoscopic surgical services offered at Penn State Health. Dr. Moyer’s clinical practice centers on pancreatic and biliary disease, inflammatory bowel disease and interventional endoscopy including ERCP, EUS, EUS-guided interventional techniques, EMR, ESD, chemoablation of cystic lesions and transluminal endoscopic surgery. Dr. Moyer also periodically staffs the free medical clinic of the Bethesda Mission in Harrisburg.
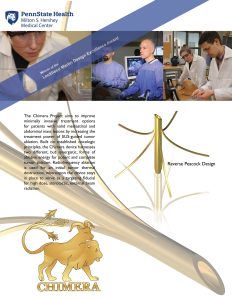
The Chimera Project is a joint effort with Penn State Bioengineering to develop a minimally invasive EUS-guided ablation device for solid abdominal and mediastinal tumors. (Click to view full image)
Research and development are the hallmarks of the interventional endoscopy group. Dr. Moyer has authored over 75 major publications, presented at numerous national meetings and received numerous awards in this area including the 2017 American College of Gastroenterology Governors Award for Excellence in Clinical Research and the 2016 Lockheed Martin Design Excellence Award.
Although Dr. Moyer’s research team has published on multiple techniques in recent years, their most significant current projects include the multicenter, randomized, prospective CHARM II trial, which is further developing the innovation of EUS-guided chemoablation of pancreatic mucinous cystic tumors. Pancreatic cysts are precancerous in a majority of cases and this innovative procedure presents a minimally invasive option to eliminate these precursors to pancreatic adenocarcinoma in appropriately selected cases. CHARM II is funded by the National Institutes of Health and is an active collaboration between Penn State Health and Indiana University. The trial is currently enrolling patients.
Along with Charles E. Dye, MD, this innovative research team is also responsible for the development of the Chimera Project, a joint effort with the Penn State Bioengineering to develop a minimally invasive EUS-guided ablation device for solid abdominal and mediastinal tumors.
Dr. Moyer is currently funded by an NIH R01 grant and the Anna Fakadej Research and Development Fund.
View Dr. Moyer’s research profile
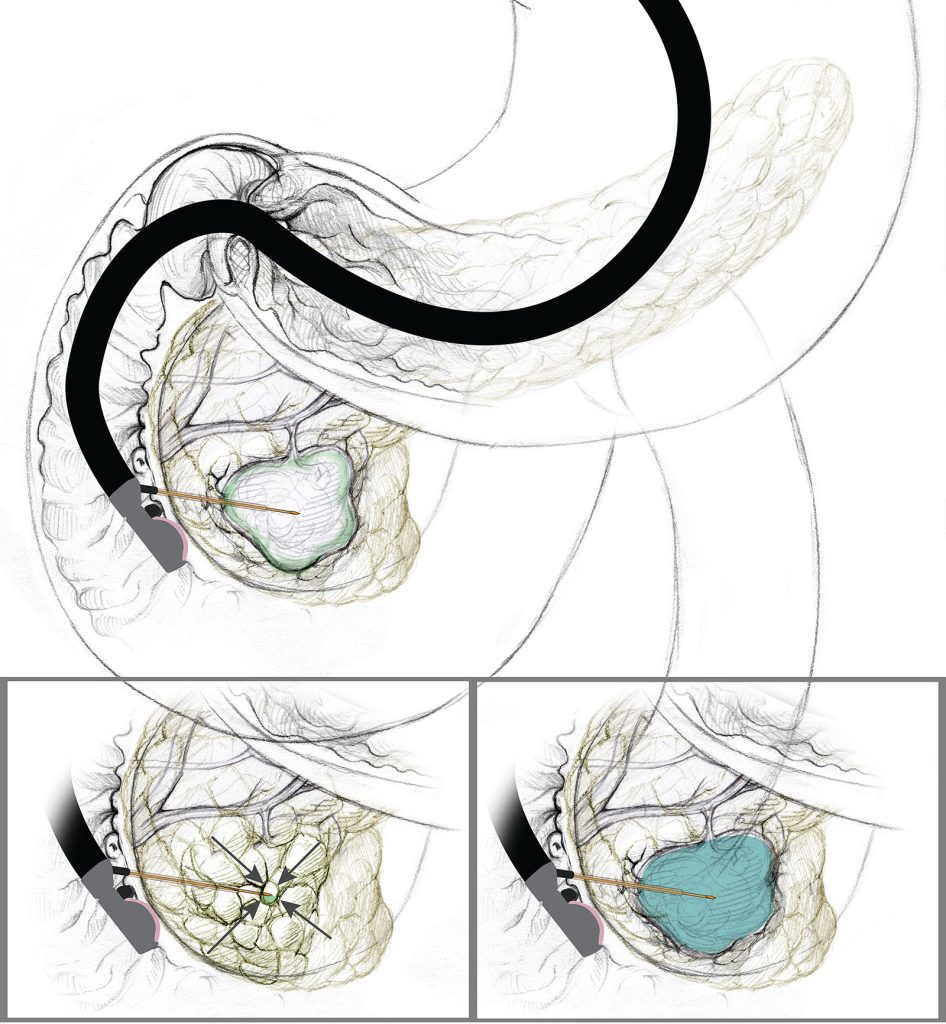
The alcohol-free, EUS-guided, cyst ablation process: The FNA needle is introduced into the center of the cystic lesion, followed by near complete aspiration of mucinous fluid from all compartments, leaving a small rim of fluid around the needle tip to prevent damaging the cyst wall. This is followed subsequently filling the cyst with an equal volume of the CHARM chemotherapy ablation admixture.
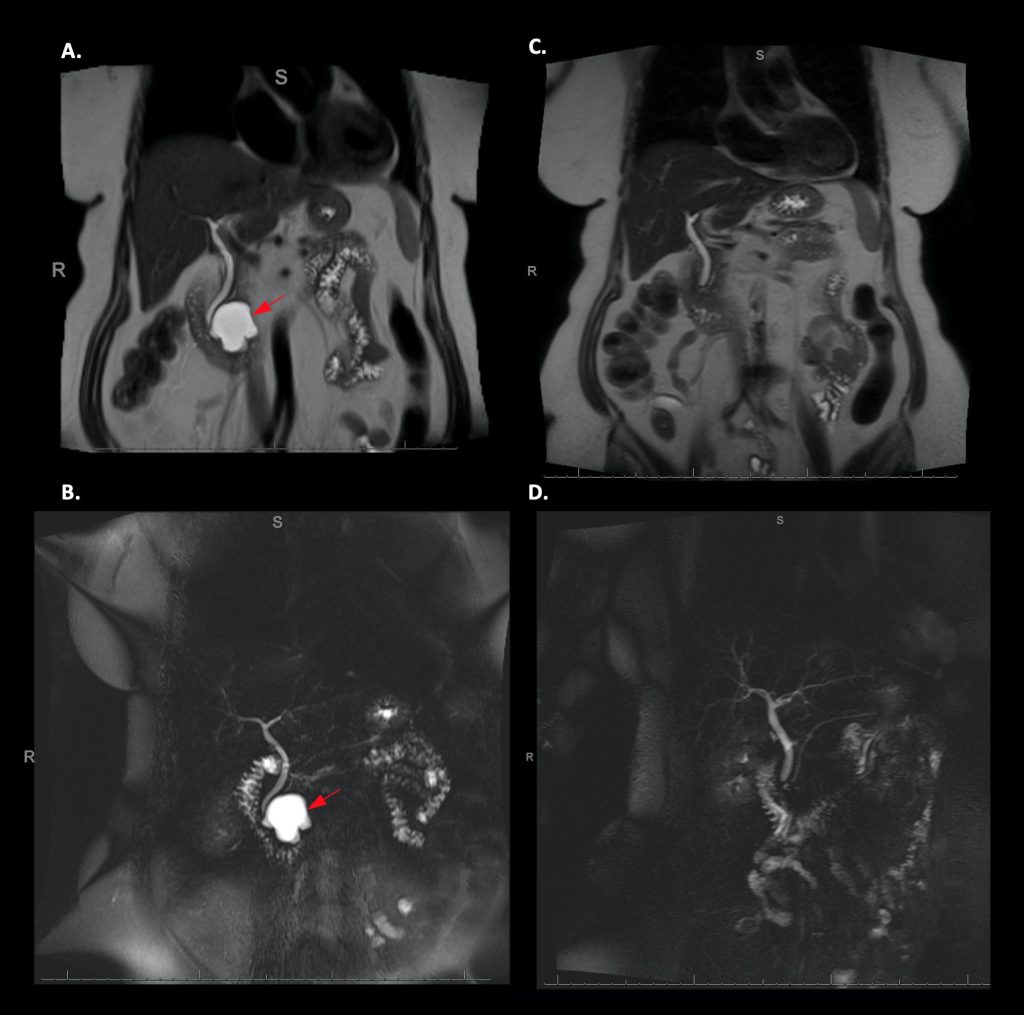
Before and after MRI and MRCP images of a patient with a 3.6cm mucinous (precancerous) pancreatic cyst participating in the CHARM Trial. Images on the right show complete resolution of the cyst after EUS-guided chemoablation using the CHARM Protocol.

Brandon Rodgers
Research Fellow
Division of Gastroenterology and Hepatology
Dr. Rodgers graduated from Virginia Tech with a Bachelor of Science in Biological Sciences and went on to medical school at Rutgers New Jersey Medical School. He then completed his internal medicine residency at the University of Maryland Medical Center in Baltimore, MD. He is now a gastroenterology and hepatology fellow at Penn State Health Milton S. Hershey Medical Center. His career interests include therapeutic endoscopy and medical education. He is specifically interested in the endoscopic evaluation and treatment of pancreaticobiliary malignancies and pre-malignant lesions.

Sydney Rhoades
Research Project Manager
Division of Gastroenterology and Hepatology
Sydney graduated from Misericordia University with a Bachelor’s in health sciences. She joined the Division of Gastroenterology and Hepatology in November 2021 to coordinate and organize the Penn State Interventional Endoscopy Group. She manages the regulatory and data management aspects of the group’s active and past research projects and works closely with the FDA, DSMC and IRB. Sydney’s primary responsibility is the NIH-funded CHARM ll clinical trial. She said that she is excited to work with other projects as well and is proud to be part of a team that is creating new innovations.

John Maynard Levenick, MD, FASGE
Associate Professor of Medicine
Division of Gastroenterology and Hepatology
Dr. Levenick is a therapeutic endoscopist with research interests in precancerous lesions of the gastrointestinal system. In collaboration with his team, he is involved in several international studies assessing different techniques to improve the outcomes of patients with large precancerous colon polyps as well as Barrett’s esophagus. Through these studies, the team is looking at safer techniques to remove large colon polyps as well as ways to reduce local recurrence of these polyps.

Abraham Mathew, MD
Graham H. Jeffries Professor of Medicine
Division of Gastroenterology and Hepatology
Penn State Cancer Institute
Dr. Mathew has a longstanding and active research program in interventional endoscopy, beginning in 2006 with innovative animal research studies on natural orifice translumenal endoscopic surgery (NOTES) with Matthew Moyer, MD, MS, FASGE, Eric Pauli, MD, and Randy Haluck, MD.
Dr. Mathew has continued to develop innovative endosurgical techniques and has multiple publications and national presentations involving NOTES, peroral endoscopic myotomy (POEM) and interesting case reports demonstrating innovative endoscopic techniques.

Wes Heinle, MPH
Research Project Manager
Division of Gastroenterology and Hepatology
Wes graduated from Robert Morris University with a Bachelor of Science in biology and a minor in chemistry. He then began working in various research roles while he completed a Master of Public Health from Thomas Jefferson University. Soon after joining the Division of Gastroenterology and Hepatology as a Research Project Manager, Wes was introduced to the CHARM II protocol. This RO1-funded work represents a large step toward pancreatic cancer prevention. Wes has expertise in REDCap, the study’s database, as well as navigating the auditing, monitoring, DSMC and FDA reporting requirements of the study.

Zvjezdana (Stella) Chroneos, PhD, CCRP
Study Coordinator
Division of Gastroenterology and Hepatology
Dr. Chroneos’s educational background and training are in basic science research. She joined the Interventional Endoscopy Research Lab team in 2021 but had been in the study coordinator role since joining Penn State Health in 2011. After moving to Ohio from her native Croatia, she earned a doctoral degree from the University of Cincinnati, in Cincinnati, Ohio, in basic sciences.
Dr. Chroneos noted that the interventional endoscopy program is a dynamic group of researchers, physicians and scientists who are creative and pioneering in treating various gastrointestinal (GI) conditions and diseases. She very excited to participate in the interventional endoscopy research program’s clinical trials that help improve GI patients’ conditions and advance science.

Allison Leisgang, DO
Internal Medicine Resident
Dr. Leisgang graduated from Clemson University with a Bachelor of Science in Biological Sciences and went on to medical school at Edward Via College of Osteopathic Medicine-Carolinas campus. There, she found her passion for gastroenterology, and is excited about joining the interventional endoscopy research team to further the field of gastroenterology. Her research interests include pancreatic cancer prevention and IBD.
Research Topics, Current Projects and Clinical Trials
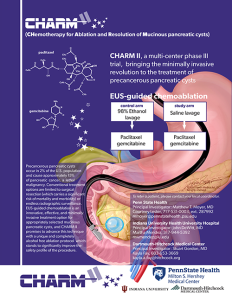
An information flyer about the CHARM study. Click to view the image larger or click on the “learn more about CHARM II” button for more information.
The interventional endoscopy group is currently recruiting for the following clinical trials.
CHARM II (Chemotherapeutic Ablation and Resolution of Mucinous pancreatic cysts)
This clinical trial centers on the investigation of treating premalignant pancreatic cystic lesions by EUS delivered chemotherapy. This National Institutes of Health-funded, multicenter, prospective clinical trial will be actively recruiting from 2019-2024.
CLIP II
A multicenter study assessing the safety and efficacy of two different ways to remove large colon polyps. Traditional resection methods involved using electrocautery to remove large polyps, but this can lead to some of the complications associated with large polyp removal. This study directly compares electrocautery to no electrocautery for removing large colon polyps and assesses endoscopic complication rates and local recurrence of polyps at the site of resection trying to determine the best and safest way to remove these lesions.
Hybrid APC assisted large polyp removal (CHUM Trial)
Large colon polyps are frequently removed using endoscopic mucosal resection techniques. Unfortunately, despite multiple different methods of removing and treating the polyps, there is up to a 20% local recurrence rate of the polyp at the previous resection site. This study looks at a novel approach to removing the polyp that reduces the chances of local recurrence.
News from Gastroenterology and Hepatology